FUNDAMENTALS OF TEXTILES
INTRODUCTION
Food, shelter and clothing are the basic needs of mankind. Clothing is made from textiles and our homes are made more comfortable and attractive by the use of textiles.
Textiles have such an important bearing on our daily lives that everyone should know something about them. From the earliest times, people have used textiles of various types for covering or modesty, warmth, personal adornment, to display personal wealth and even for biomedical and technical purpose.
A study of textiles will show, for example why certain fabrics are more durable and serviceable for specific purposes. Complete knowledge of textiles will facilitate an intelligent appraisal of standards and different qualities of textile products.
KEY WORDS:
Textiles: The word textile comes from the Latin term “Textere” meaning to “Woven”. Today the word textile is more generalized and refers to the product made from fibres.
Fiber: A fiber is defined as any product capable of being woven or otherwise made into a fabric. It may be thought of as the smallest visible unit of textile production or a fiber can be defined as a pliable hair like strand that is very small in diameter in relation to its length. Fibres are the fundamental units or the building blocks used in the making of textile yarns and fabrics.
Yarns: Yarns are produced by twisting or spinning of the textile fibers
Fabric: Fabric is a planner structure produced by interlacing or interlooping of yarns.
REASONS FOR STUDYING TEXTILES
- A study of Textiles will show, for example why certain fabrics are more durable and therefore more serviceable for specific purpose.
- It will explain why certain fabrics make cool wearing apparel.
- Complete knowledge of textiles will facilitate an intelligent appraisal of standards and brands of merchandise and will develop the ability to distinguish quality in fabrics.
- The customers get idea about how to buy and what to buy.
TERMINOLOGY USED IN TEXTILES
- Filament: Long continuous fiber strands of indefinite length measured in yards or meters.
- Staple: These fibers measured in inches or centimeters and range in length from ¾ of an inch to 18 inches.
- Abrasion resistance: It is the ability of a fiber to withstand the rubbing or abrasion it gets in everyday use. I t contributes to fabric durability.
- Absorbency or Moisture Regain: Is the amount of water a bone dry fiber will absorb from the air under standard conditions of temperature (70 Degree F) and moisture(65% relative humidity).
- Flexibility: Is the ability of a fiber to blend easily.
- Chemical reactivity: Is the effect of acids, alkali, oxidizing agents, solvents.
- Cohesiveness: Is the ability of fibers to cling together during spinning. Not important in continuous filament.
- Cover: The ability to occupy space for concealment or protection.
- Creep: It is delayed elasticity. Recovers gradually from strain.
- Dyeability: It is the fibers receptivity to coloration by dyes.
- Elastic recovery: Is the ability of fibers to recover from strain.
- Elasticity: Is the ability of a stretched material to return immediately to its original size.
- Electrical conductivity: Is the ability to transfer electrical charges.
- Elongation: Is the ability to be stretched, extended, or lengthened. It varies at different temperatures and when wet or dry.
- Feltability: It refers to the ability of fibers to mat together.
- Flammability: Is the ability to ignite and burn.
- Hydrophilic: Fibers are able to absorb water easily or water loving.
- Hydrophobic: Fibers that have difficulty in absorbing water and are only able to absorb small amounts are called hydrophobic. Example: All man made fibers expect rayon.
- Hand: It is the way a fiber feels: silky, harsh, soft, crisp, dry.
- Heat conductivity: Is the ability to conduct heat away from the body
- Hygroscopic: Those fibers, which absorb the moisture from air.
- Heat sensitivity: Is the ability to soften, melt, or shrink when subjected to heat.
- Luster: Is the light reflected from a surface. More subdued than shine; light rays are broken up.
- Loft or compression resiliency: Is the ability to spring back to original thickness after being compressed.
- Pilling: Is the balling up of fiber ends on the surface of fabrics.
- Static Build Up: Problems such as sparks and clinging clothing occur with the build – up change on the fiber surface.
- Thermoplastic fiber: Those fibers, melts or soften when heat is applied.
- Specific gravity and density: These are measures of the weight in grams per cubic centimeter, and specific gravity is the ratio of the mass of the fiber to an equal volume of water at 4 degree centigrade.
- Stiffness or rigidity: Is the opposite of flexibility. It is the resistance to bending or creasing.
- Solubility: It is the test used to identify the textile fiber by dissolving them in the respective solutions.
- Tensile Strength: Is defined as the ability to resist stress and is expressed as tensile strength (pounds per square inch) or as tenacity (grams per denier).
- Wicking: Is the ability of a fiber to transfer moisture along its surface.
- Sunlight resistance: Is ability to withstand degradation from direct sunlight.
- Ageing Resistance: Resistance from deterioration of textiles resulting from exposure to destructive elements encountered in normal everyday use such as sunlight, heat, moisture or oxygen.
- Moth Resistance: Resistance from moths, which is generally attracted by wool, as it is a living natural fiber or protein fibers.
- Mildew Resistance: Resistance from the Mildew, mold, fungus, and rot all which are formed on the fabrics by exposure to warm, moist atmosphere or soaps and sizing used in processing, which become food for vegetable organisms.
- Resistance from Microorganisms: Resistance of textile fibers from any of the microorganisms for the hygienic purpose.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- http://bieap.gov.in/Pdf/CGTPaperII.pdf
- https://www.textil.eu/en/articles-18/
- https://www.ballyribbon.com/glossary/
- https://en.wikipedia.org/wiki/Glossary_of_textile_manufacturing
ASSIGNMENT
- List the various products made of textiles in our day to day life.
- What are the uses of textiles in our life? List them.
- What are the textile products we use other than dress/clothes.
CLASSIFICATION OF TEXTILE FIBRES
- INTRODUCTION
The textile industry uses many fibers as its raw materials. As a result of the development of new fibers, difficulties arise in textile industry in terms of identification, classification. Hence, classification of textile fibers was compounded by the trained manufactures to identify each of the fibers with the different trademarks.
Textile fibers are classified according to the source and the length of the fibers.
- CLASSIFICATION OF FIBERS
Textile fibers are classified in the following ways
- According to the Source
- Length of the fiber
2.1 ACCORDING TO THE SOURCE:
According to the source from which textile fibers are obtained, fibers are broadly classified in two ways viz,
- Natural
- Manmade
- Synthetic
2.1.1. NATURAL FIBERS:
Natural fibers are further divided into following three categories. They are,
- Vegetable fibers or cellulosic fibers
- Animal fibers or Protein fibers
- Mineral fibers
a. Vegetable fibers ‘or’ Cellulosic fibers:
As the name indicates these fibers are obtained from vegetable source i.e. plants and cellulosic matter is the base for all these fibres.
This category includes the most important textile fiber – Cotton, which makes up nearly 50% of total fibers used in world (by weight). Hence it is considered as major fiber. This category also includes other minor fibers such as Linen, Jute, Pineapple, etc.
b. Animal Fibers ‘or’ Protein fibers:
There are several animal fibers each obtained from a different source, but only two are recognized as major textile fibers – Wool and Silk. They make up small proportion in the market (by weight) but a much higher proportion by value. Other minor fibers in this category are Mohair, Cashmere, Angora, and Camel hair.
c. Mineral Fibers:
Asbestos is a natural fiber obtained from varieties of rock. It is a fibrous form of silicate of magnesium and calcium containing iron, aluminum and other minerals. It is an acid proof, rustproof, flameproof fibre. However, the use of asbestos is now rapidly declining following the discovery of health risks from asbestos dust.
2.1.2. MANMADE FIBERS:
These refer to those fibers that are not naturally present in nature, but are synthesized.
a. Cellulosic Source:
These are the fibers manufactured from a natural polymer Cellulose that is obtained from wood. These are further divided into:
i. Regenerated fibers: The starting product is cellulose, which is dissolved in sodium hydroxide and the viscous solution is extruded through spinneret into dilute H2SO4.
ii. Modified Regenerated: The raw material here is also cellulose, but these fibers are modified chemically so that polymer can be dissolved in an organic solvent and extruded into hot air which evaporates the solvent.
b. Protein Fibers:
These are made from the protein source but are not being manufactured at present. They include Soya bean and Azlon.
c. Mineral Fibers:
These fibers are glass, steel and carbon, all of which are found in industrial end uses viz., Glass is used for low cost reinforcement plastic for ships, cars and thermal and electrical insulation etc.,
Steel is reinforced rubber in tyres and belts for filters where chemical resistance is important.
Carbon fibers are used where high performance is required i.e. aircraft’s parts, tennis and squash rackets etc.
2.1.3. SYNTHETIC FIBERS:
The term synthetic means that the polymer is entirely man made from chemicals. These group fiber properties are dependent upon their chemical compositions and kinds of molecular orientation. The group includes three major fibers and several minor ones. The major ones include Nylon (polyamide produced in U.S.A in 1938), Polyester (produced in 1953), Acrylic (produced in 1948).
2.2.CLASSIFICATION OF FIBERS BY LENGTH:
According to length, fibers are classified into two types:
- Staple fibers
- Filament fibers
2.2.1. STAPLE FIBERS:
Natural fibers are short length fibers which measure in inches or fraction of inch Ex: ¾ to 18 inches. Expect silk, all other natural fibers are staple fibers. Manmade fibers are made in filament form but can be cut into short staple lengths for particular end uses.
2.2.2. FILAMENT FIBERS:
Long fibers those measured in yards or meters are known as filaments. Silk and all man-made and synthetic fibers are filaments.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- http://gpktt.weebly.com/classification-of-textile-fibers.html
- https://clothingindustry.blogspot.com/2017/12/classification-textile-fibers.html
- https://www.youtube.com/watch?v=76xEqJXhqrk
- http://bieap.gov.in/Pdf/CGTPaperII.pdf
ASSIGNMENT
- Collect textile fibers from their products
- Identify textile fibers by their length
- Collect natural fibers in their original form
PROPERTIES OF TEXTILE FIBRES – Primary
- INTRODUCTION:
The second step in the process of learning textiles is to know how to qualify a material for a specific use. In order to do so the product must possess some essential characteristics or properties. It is generally agreed that the essential qualities or primary properties of a fibre like length, strength, flexibility or pliability, cohesiveness or spinnability and uniformity are adequate. Other properties may be desirable in all fibres but they are not essential. So they can be termed as secondary properties. These may improve the quality of textile fibres and thus get the consumers’ satisfaction.
- PROPERTIES OF TEXTILE FIBRES:
The properties of the fibers can be divided into:
- Primary properties
- Secondary properties
- Thermal properties
- Chemical properties
- Biological and other properties
2.1 PRIMARY PROPERTIES
Primary properties of the fibers include:
- Length
- Strength or Tenacities
- Flexibility or Pliability
- Cohesiveness or Spinnability
- Uniformity
2.1.1 Length:
Textile fibres are available in different lengths. Filaments are long continuous fibres of indefinite length measured in yards or meters. Staple fibres are short fibres measured in inches or centimeters and range in length from ¾ ” to 18″. All natural fibres except silk are staple fibres. Man-made and synthetic fibres are all filament fibres. Sometimes filament fibres are cut into staple length. Several thousands of filaments are taken in the form of a loose rope or strand, often made crimpy and are cut to produce staple fibres ranging in length from 1″ to 5″. The rope of fibres is also refered to as ‘Fibre tow’.
Fibrous materials must possess greater length than the diameter. This is referred as length to width ratio. A minimum ratio of 100:1 is considered essential. Majority of the fibres have greater length than the diameter.
2.1.2 Strength or tenacity:
Strength is the second primary property of all textile fibres. In order to be serviceable, all fibres must possess this quality. The strength must be adequate for processing or spinning into a yarn and further making into a fabric. Fibres may vary in strength and the strength within a fibre may not be uniform throughout. It depends upon mainly the molecular structure of fibres.
The strength of a fibre is defined as the ability to resist stress and is expressed in grams per denier (finesses of fiber). It may vary from fibre to fibre. Strength can also be measured in pounds / sq.inch. This is referred as ‘tensile strength’. The strength of a fibre can never be confused with strength of yarn or fabric since it is possible to produce very strong and durable materials from weak fibres.
A strong fibre is durable, has a better tear strength and resists sagging and pilling. The tenacities of some selected fibres of consumers’ interest are given below.
Fibre Tenacities
Under Standard Conditions (70 Degree Fahrenheit & 65% RH)
- Cotton – 0
- Silk – 5
- Wool – 5
- Rayon – 5 to 2.4
- Acetate – 2 to 1.5
- Nylon – 5 to 5.9 (High tenacity fibres– 5.9 to 9.2)
- Polyester – 4 to 7.8
- Glass – 0
Glass ranks first in tenacity than the other fibres. Next is Nylon and Polyester. Some of these fibres loose or gain strength when wet. A good example for loss of strength during wet condition is Rayon and for gaining strength is cotton.
2.1.3 Flexibility or Pliability:
Certain degree of flexibility or pliability is necessary for a fibre to be used as a textile fibre. A textile fibre needs to be bendable. For example a glass rod cannot be bent without breaking, but a glass filament can be bent easily. This property is essential to create yarns and fabrics that can be creased, have the quality of drapability, ability to move with the body and should allow for the free movement and also be comfortable. A stiff fibre will make stiff fabrics, which cannot be used comfortably.
2.1.4 Cohesiveness or Spinnability:
Cohesiveness is the ability of the fibres to stick together during spinning. The cohesiveness in fibres may be due to the longitudinal contour or the cross sectional shape that enable them to adhere together. The surface or the skin structure of the fibre may also influence cohesiveness. For example, wool fibre possesses scales on the outer skin of the fibre which help in interlocking fibres while spinning. If the surface or shape of a fibre does not contribute for cohesiveness, the same can be compensated by using filament yarns. As filaments are present throughout the length of the yarns, there is little necessity of having the ability to stick. So this cohesiveness is often conveniently replaced by spinning quality. Polyester is having the lowest cohesiveness but it can be made into staple yarns by using less percentage of cotton and later burning it through carbonising process.
2.1.5 Uniformity:
In order to produce fine yarns, uniformity in the raw material is required. Fibres that are used to produce yarns need to be similar in length and width, in spinning quality and in flexibility. All man-made and synthetic fibres are uniform since they are made through artificial processing. But in case of natural fibres, it is not so. Fibres differ is many aspects, and so it is not possible to produce very fine materials in natural fibres unless some extra processing is done. The yarns composed of uniform fibres are smooth and even.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- Wynne A (1997) Textiles The Motivate Series, Macmillan
- http://bieap.gov.in/Pdf/CGTPaperII.pdf
- http://textilelearner.blogspot.com/2012/02/properties-of-fiber-properties-of.html
ASSIGNMENT
- Identify the important properties of the textile products
- List any five important primary properties we need for clothes and why?
- Identify strongest fiber
PROPERTIES OF TEXTILE FIBRES – SECONDARY
- INTRODUCTION:
Secondary properties include: Physical shape, lustre, density or specific gravity, absorbency, electrical conductivity, elasticity, elastic recovery, pilling, hand, feltability. The following is the detailed information about the characteristics of textiles fibres.
- SECONDARY PROPERTIES:
- Physical Shape
- Density
- Lustre
- Absorbency
- Elasticity
- Abrasion Resistance
- Hand
- Pilling
- Loft and Resiliency
- Static Electricity
- Feltability
2.1 Physical Shape:
The physical shape of the fibre is an important factor in determining many of its properties. It includes the surface contour (smooth, rough, serrated), the shape of the cross section and the width and length of the fibre. The shape of the cross section influences certain factors such as lustre, body and hand. The surface contour in turn influences cohesiveness, resiliency, loft and thickness. It contributes to resistance to abrasion, pilling and comfort factors such as absorbency and warmth. The cross sectional shape can be changed for all artificial fibres unlike natural fibres as the fibres are molded though spinnerets.
2.2 Density:
Density is the mass of a unit volume of material. It is expressed as gms/cubic cm or pounds per cubic foot. The specific gravity of a fibre indicates the density relative to that of water at 4oC. All textile fibres are heavier than water except olefin fibres. Only these fibres float on water. Cotton, wool fibres are heavy and nylon is comparatively lighter. The lower the density of fiber the more the covering power they possess. A pound of wool and a pound of nylon weigh the same but the fibres are more in nylon than in wool. High density results in heavy fabrics, low density results in light weight fabrics.
A light weight fibre helps a fabric to be warm without being heavy. Acrylic fibres being light comparatively are wool like in appearance and are used extensively instead of wool to produce light weight sweaters and blankets.
2.3 Lustre:
Lustre is the amount of light reflected from a surface. It is more subdued than shine. Light rays are broken up into many short rays unlike the shine in which the light ray is reflected back wholly without any breaks. The lustre is due to smoothness, fibre length, flat or lobal shape. It determines the fibres natural brightness or dullness. The natural fibre silk has the high lustre and cotton is the dullest natural fibre. All man-made fibres are produced with controlled lustre. It is not always desirable to produce bright fabrics. So the lustre is controlled by the addition of pigments such as titanium dioxide in spinning solution. The lustre in natural and man-made fibres can also be improved by various finishing techniques. For example the lustre in cotton is improved by mercerization.
2.4 Absorbency:
Generally textile fibres have certain amount of water as an integral part of the fibre. All most all textiles fibres are naturally hygroscopic (i.e they pick up moisture from air). But the amount of moisture the fibres absorb may differ. Absorbency is the ability to take in moisture and moisture regain is the percentage of moisture a bone-dry fibre will absorb from the air under the standard conditions of temperature and moisture. Fibres that absorb water easily are known as hydrophilic (water loving) fibres. Natural protein and vegetable fibres, rayon and acetate are hydrophilic fibres. Fibres that have difficulty in absorbing water are known as hydrophobic fibres.
Many synthetic fibres are hydrophobic in nature. The absorbency of glass fibre is ‘0’. Absorbency is an important factor in all textile fibres especially those which are used for apparels as it influences many other fabric properties such as comfort, warmth, water repellency, static build up, dyeability, shrinkage, wrinkle resistance etc. It is easy to wash a hydrophobic fabric as it does not absorb stains and it dries quickly.
Among the textile fibres the natural protein fibres silk and wool are the most absorbent of all fibres. Next comes the natural and man-made cellulosic fibres.
The absorbency of a textile fabric is controlled by the type of yarn and fabric construction and also by finishing. For example: in cotton, the absorbency is increased by kier boiling, mercerization and napping. Pile construction increases the area of absorption.
2.5 Elasticity:
Elasticity is defined as the ability of fibres to return back to original shape after being stretched. Elastic recovery is the ability of fibres to return from strain and is expressed in percentage. If a fibre returns to original length after stretching to a specified length, it is said to have 100% elastic recovery.
Elasticity is required in fabrics when subjected to stretch during wear. This property is influenced by the side chains and cross linkages between the molecules. If strong bonds are present in between chains of molecules, the fibre tends to return to its original length. If the bonds are not strong it can’t recover to its original length but takes up the new shape. Thus creases appear on the material. Some fibres show immediate elastic recovery, and some fibres may show delayed elastic recovery. For example, the creases on a silk material disappear if hung overnight. Wool, silk, viscose and nylon are having good elasticity. Cotton and acetates have poor elastic recovery. Polyester has moderate elongation but has good elastic recovery. It is apparent that both the elongation and elastic recovery are considered together in evaluating fibres, yarns and fabrics.
2.6 Abrasion Resistance:
It is the ability of fibres to withstand the rubbing or abrasion it gets in everyday use. All fabrics irrespective of the end use are subjected to rubbing of some kind during wear. The fabric has to withstand rubbing, otherwise the fabric will show signs of damage and become unsightly. The resistance may be due to the tough outer layer and flexible molecular chains of the fibre. The size of the yarn also influences the abrasion resistance. Thick yarns resist abrasion than thin yarns. Yarn uniformity is also important as irregular yarns are abraded more easily than uniform yarns. Smooth fabrics with compact yarn arrangement are less susceptible to damage by abrasion than those with irregular surface in the low count.
Nylon has excellent resistance and acetate and glass have very poor abrasion resistance when compared to silk & wool. Cotton has better abrasion resistance. This is an important property, as it influences the durability and increases the resistance to splitting.
2.7 Hand:
Hand is the way a fibre feels. It can be only detected by feeling it in between fingers. The hand varies due to the cross sectional shape, the length and diameter, the flexibility, the compressablity, resilience, surface contour of the fibres, surface friction and thermal characteristics of fibres .
The hand and drape of a fabric are inter dependent. The hand of a fabric may vary from very pliable to very stiff, from very soft to very hard, from very limpy to very springy, from rigid to high degree of stress, form very smooth to very rough, from slippery to harsh, from very cool to very hot and from wet to dry.
The hand of a yarn and fabric should not be confused with the hand of a fibre. It is possible to produce smooth yarns from rough fibres and vice versa.
2.8 Pilling:
Ball like structures are often observed on polyester and nylon materials after few washes which make the material unsightly. Pilling is nothing but the balling up of fibre ends on the surface of fabrics. It is one of the disadvantages of staple fibre fabrics. In natural fibres the balls cut away from the fabric easily but synthetic fibres are so strong that they do not break away rapidly from the fabric. So the strength of fibres is a basic factor in the problem of pilling. Pills usually occur in areas that are abraded or subjected to abrasion during wear. Usually at the armpits of garments and back and lower edge of sarees, pilling can be seen. It can be made better by removing pills. But it is almost impossible to remove pilling from synthetics unless it is given singeing finish. In this the fabric passes through gas flames, so that the balls are burnt off. In order to inhibit the formation of pills on materials, they are given special finishes known as anti pilling finishes.
To prevent pilling close fabric construction is recommended. Tightly twisted yarns and longer staple fibres are helpful in preventing pilling. Fulling of wool, resin finishes on cotton are anti pilling finishes.
2.9 Loft and Resiliency:
Loft is the ability of a fibre to spring back to original thickness after being compressed. Resiliency is the ability of a fibre to bounce back to shape following compression, bending or similar deformation. Wool and silk fabrics are more resilient. They can be deformed, crushed or wrinkled during wear but they come to shape upon hanging. Elastic recovery is an important factor while evaluating the resilience of a fibre. Usually good elastic recovery indicates good resiliency.
2.10 Static Electricity:
This is the electricity produced by the friction of a fabric against itself or some other object. If a fabric is better conductor of electricity, it conducts away the electricity that is produced. But if the material is not a good conductor, the electricity produced cannot be conducted away, but it tends to pile up on the surface of the fabric. It the material comes in contact with a good conductor, a shock or transfer occurs. It may produce sparks, in gaseous atmosphere, it may give explosions. So it is a hazard in places where materials which are highly inflammable are present. So the use of synthetics is prohibited in operation theatres. Static electricity rapidly develops in cold and dry atmospheres. After wearing synthetics for few hours, it is better to wipe the garments with a wet towel. It carries away the electricity produced. Static electricity makes the fabric to cling to the body of the wearer. It attracts more dust and thus gives unsightly appearance. Fabrics cling to the machinery & thus cutting and stitching of garments is made difficult.
Antistatic finishes are given to fabrics in order to inhibit the piling up of static electricity on fabrics. But this is washed off after few washes.
2.11 Feltability:
It is the ability of fibre to mat together. Using this property, it is possible to produce fabrics without the complicated processing of spinning and weaving. These are termed as non – woven felted materials. Some rug materials, carpet materials and apparels are produced by felting. The ability of wool to coil together, interlock & shrink when subjected to heat, moisture and pressure is responsible for felting of wool fibres. In fact the other fibres are also felted by using a suitable adhesive.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- Wynne A (1997) Textiles The Motivate Series, Macmillan
- http://bieap.gov.in/Pdf/CGTPaperII.pdf
- http://textilelearner.blogspot.com/2012/02/properties-of-fiber-properties-of.html
ASSIGNMENT
- Identify the different properties of the textile products
- List any five important secondary properties we need for clothes and why?
- Identify any five important properties that help in providing comfort to the wearer
THERMAL, CHEMICAL, BIOLOGICAL PROPERTIES AND MOLECULAR STRUCTURE OF TEXTILE FIBRES
- INTRODUCTION:
After the primary and secondary properties thermal behavior, the resistance to biological organism and resistance to chemicals and other environmental conditions are also important to study especially for providing hygienic clothes.
- THERMAL PROPERTIES:
The thermal behaviour of fibres is also an important factor for determining their performance and care. The burning characteristics of fibres help in fibre identification.
Among the textile fibres which are in use, some are heat sensitive and some are not heat sensitive. Heat sensitivity is the ability to soften, melt or shrink when subjected to heat like plastic. This category of fibres is also known as thermoplastic fibre. All synthetic & acetate are thermoplastic fibres. Others which are not heat sensitive are non-thermoplastic fibres. The heat sensitivity of a fibre may be due to the vibration of molecules in the fibre by heat. These fabrics need to be finished after washing, at safe, lower and recommended temperatures.
We feel comfortable when we wear some garments and we feel hot with some other garments this is mainly due to the heat conductivity of fabrics. Heat conductivity is the ability of fibres to conduct away heat from the body. This is also an important factor since; it contributes the use of certain fabrics in a particular season.
- CHEMICAL PROPERTIES:
The reaction of fibres to various chemicals is helpful in use and care of fabrics, chemical reactivity is the effect of acids, alkalies, oxidizing agents and solvents. The fibres react differently to various chemicals and these are explained under each fibre.
- BIOLOGICAL AND OTHER PROPERTIES:
Biological & other properties such as ageing resistance, sunlight resistance, resistance to moths, mildew and microorganisms play an important role in determining the performance of fabrics in use and care. All these are discussed in detail under each fibre.
- MOLECULAR STRUCTURE OF FIBRES:
As any other matter, textile fibres are also having chemical structure. The chemical structure of fibres is different from one another. For example: Cotton fibre is made up of cellulose which consists of hydrogen, carbon & oxygen. The way in which the chemical elements are arranged is what makes cotton different from polyester which has the same chemical constituents. The chemical structure affects fibre properties such as strength, elongation, resiliency, density, moisture content, sunlight and ageing resistance, dye absorption and electrical behaviour.
The fibre morphology refers to the molecular arrangement of fibre. The molecules in fibres are in the form of chains. These are known as polymers. Polymerization is the process of joining together small molecules known as monomers. The longer the chains, the higher the degree of polymerization.
The arrangement of molecules in these fibres can be described in terms of molecular orientation and amorphous regions. When the molecules of fibres are parallel to each other and also parallel to the longitudinal axis of the fibres, the arrangement is said to be highly oriented structure. If the molecules are arranged in haphazard way or at random, it is termed as amorphous or low oriented structure. A crystalline structure occurs when the fibre molecules are parallel to each other but not necessarily parallel to the fibre axis.
In a single fibre, it is common to find both amorphous & crystalline regions and fibres vary in the proportion of oriented, crystalline and amorphous regions.
The molecular arrangement of natural fibres is difficult to change except in cotton where the molecules tend to decrystallise by mercerization finish.
The man-made & synthetic fibres when extruded through the spinnerette consist of only random arrangement but when they are stretched they become thin and tend to take oriented structure. It improves the strength, elongation, moisture absorption, abrasion resistance & dyeability of fibres.
The properties like elasticity, strength etc are also dependent on the strength of the bonds between molecules. These molecular chains are held together by cross links or inter chain attractions or bonds such as Hydrogen bonds & Vander walls forces. Hydrogen bonds are stronger than vanderwalls forces even though both are found in crystalline arrangements. Hydrogen bonding occurs when the positive hydrogen atoms show attraction towards negative oxygen or carbon present in another chain. Thus the molecular structure of fibres is an important factor that affects the properties of fibres.
The molecules are held together in a chain like formation within a macromolecule by strong electronic force known as valency bonds. For example cellulose which is the base of cotton is formed by the polymerization of small, simple molecules into a larger complicated macromolecule.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- Wynne A (1997) Textiles The Motivate Series, Macmillan
- http://bieap.gov.in/Pdf/CGTPaperII.pdf
- http://textilelearner.blogspot.com/2012/02/properties-of-fiber-properties-of.html
- http://textilelearner.blogspot.com/2011/07/termal-properties-of-textile-fibers.html
- http://textilestudy365.blogspot.com/2017/10/chemical-properties-of-textile-fibers.html
- https://study.com/academy/lesson/textile-fibers-definition-properties-types.html
ASSIGNMENT
- Identify the different properties of the textile products
- List any two important properties which are important in laundering clothes
- List the parts of textile fiber structures
- List the bonds present in textile fibers
COTTON FIBRE
- INTRODUCTION
Cotton is a seed hair obtained from the pod of the cotton plant that belongs to malvaceae family and class Gossypium. It is classified as a natural, cellulose, seed, staple fiber measuring 10-65mm in length and white to beige in color in their natural state. Cotton fiber grows from the surface of seeds in pods, or bolls, of a bushy mallow cotton plant hence it is called as seed hair. It is composed basically of a substance called cellulose, on burning cotton smells of burning paper and leaves a grey fluffy ash. As cotton occupies the 50% of consumption of fibers by weight in the world, it is called as the king of all fibers.
The origin of the fibre is unknown. Archaeologists confirm that cotton was grown in Egypt about 12000 BC. Evidences of growing cotton in India about 3000 BC are existing. Most of the earlier records also confirm that India was major country that grew cotton and exported to other countries. The varieties of cotton that were grown in Egypt were very fine compared to the rest of the world and considered as good quality. The term ‘Egyptian Cotton’ used even today indicates fine quality.
- CULTIVATION OF COTTON
Cotton is mostly grown in USA, India, and Egypt. Cotton. It requires 200 days of continues warm weather with adequate moisture and sunlight, frost is harmful to the plant. March and April months are suitable for plantation.
Cotton is cultivated in a warm humid climate. Black soil is considered best as it can hold moisture well. The cotton seeds are sown in fields around 3-4 feet apart in parallel rows. Irrigation is compulsory for plants growth. The plant grows to its full form within 80-1000 days and blossoms appear on plants. When open, it is creamy white in color which turns into pink within a day. The pods appear within 50-80 days and after attaining maturity, it bursts open and exposes the white fleece. Picking of cotton is done manually or by use of machine. Hand picking is very common in labour intensive countries and machines are employed in all western countries. The quality of cotton largely depends on picking operation. If the fleece alone is picked up carefully avoiding the burs and dried leaves, cotton will have good quality and is priced high. Of more impurities are present in cotton, it needs more cleaning, thus it is priced low.
- PROCESSING OF COTTON
After cotton is picked, it is taken to a ginning mill where the fibre is separated from its seed. Generally around 4,000 fibres emerge from a single cotton seed. Fine variety cotton can yield as much as 20,000 fibres from each seed. When cotton arrives at ginning mill, it contains seeds burs, leaf fragments, dirt etc. which should be removed before it is sent for spinning. Cotton gin, a machine invented by Whitney in 1974 is capable of separating cotton fleece from its impurities. It has rows of revolving saw toothed bands that pull the fibre away from the seeds and the impurities. Cotton seeds are important by-products from which oil is extracted. Cotton free from major impurities are baled and sent to spinning factories.
4. MANUFACTURING OF COTTON
4.1. Hand Made Cotton:
The tools and appliances used by the cotton weavers consist of spinning wheel (charka) spindle (takli) and a bow shaped beater (dhun). The threads then formed are wound on a bamboo reel and from which warp of the handloom is set to weave the fabric
4.2. Machine made Cotton:
Manufacturing by machine involves the following steps;
- Preparation: The fibers are first removed from the seeds, leaf fragments, dirt and other materials. The seeds are removed by the cotton gin.
- Forming the laps: In this step dirt in the cotton is removed and fibers into a soft roll or lap. Then several laps are combined into one.
- Carding: The fibers are drawn together to form a loose rope or sliver.
- Doubling: Slivers are combined.
- Combing: It is continuous and refinement of the carding process. These are free from woody stalk, used for finer quality fabrics.
- Drawing: Slivers are combined and smoothened, stretched that could cause too many variations if the slivers are feed singly. The slivers given first twist and wound on to bobbins.
- Roving: The bobbins are placed on the roving frame where further drawing and twisting takes place, until the cotton stock is about a pencil lead in diameter.
- Spinning: Done on the spinning frame where the stock passes through sets of high-speed rollers and gives the yarn of desired thickness.
- Weaving and dyeing: Any variety of weaves can be used for the cotton. Dyes are applied to raw cotton at fiber, yarn or fabric stage.
- Finishing: Both performance finishes and functional finishes are given to the cotton.
5.PROPERTIES OF COTTON FABRICS
5.1. PHYSICAL PROPERTIES
5.1.1. Structure
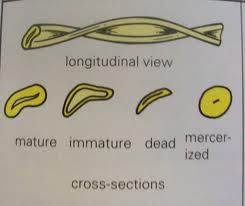
Cottonfibre is a cellulosic unicell that shows a base and tapering edges. It is a staple fibre ranging from ½ ” to 2 ½ ” in length. The diameter ranges from 12 to 20 micrometres. Cotton unicell shows outer cuticle (1), primary wall, secondary wall and a central canal called lumen (5). It is flat ribbon like with twist under microscope called ‘convulsions’. Matured fibres show narrow lumen and immature fibres show large lumen. The cross sectional shape of cotton fibre is similar to a kidney shape. Cotton fibre before ball opening exhibits round cylindrical shape but when exposed to outside atmosphere the cell sap dries up and makes it to flatten and loose its luster. About 65-70% of cotton fibre is crystalline and 35-30 % is amorphous.
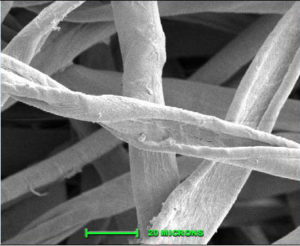

5.1.2. Strength
The strength of cotton fibre is regarded as medium and the tenacity of cotton ranges from 3.0 to 5.0 grams per denier. One unique feature of cotton is its increase in strength when wet. When wet, it shows the strength equal to 110 to 120 % of its dry weight. The tenacity of cotton is increased when it is mercerized.
5.1.3. Elasticity
Cotton fibre has low elongation due to its crystalline structure. Around 3-7 % elongation is found. Around 75 % of elastic recovery is recorded at 2 % elongation. The elastic recovery reduces with increase in percent of elongation. At 5 % elongation, cotton recovers only 50%
5.1.4. Resiliency
The resiliency of cotton is low. Cotton fabrics wrinkle very easily and require ironing after washing. Appropriate finishing process improves wrinkle resistance of cotton.
5.1.5. Abrasion resistance
Cotton shows medium resistance to abrasion.
5.1.6. Moisture Regain:
Cotton is a hydrophilic fibre. The moisture regain of the fibre is 8.5 % under standard conditions. Cottons are highly comfortable as summer wear due to high absorbency and good heat conductivity.
5.1.7. Dimensional Stability:
Cotton fibres are stable and do not shrink or stretch as they are unicellular. Fabrics may show shrinkage due to tensions during fabric construction. However, it needs pre-treatment to control shrinkage in use.
5.1.8. Thermal Properties:
Cotton fabrics are good conductors of heat and thus regarded as cool fibre. Under dry conditions cotton can absorb the body sweat and keep the wearer comfortable. Light weight, open structured cotton fabrics are highly comfortable during humid weather. Cotton being cellulosic fibre does not melt but only scorches at high temperature of around 2500C as it withstands moderate temperatures, it can be ironed safely.
Cotton fibre burns slowly in flame and continues to burn after removal from flame as it supports combustion. It gives paper smell and leaves a small amount of fluffy ash as residue.
5.2. CHEMICAL PROPERTIES:
5.2.1. Effect of alkalies:
Cotton is highly resistant to alkalies. It can withstand all alkaline reagents and soaps. Alkalies such as sodium hydroxide and ammonia are used for finishing of cotton to impart desirable qualities.
5.2.2. Effect of acids:
Cotton’s resistance to acids is inferior to its resistance to alkalies. It shows good resistance to dilute organic acids such as acetic and citric acids. Prolonged exposure may tender cotton. Dilute or strong mineral acids such as sulphuric acids will destroy cotton
5.2.3. Effect of bleaches:
Cotton can withstand chlorine and perborate bleaches and not easily damaged unless it is very strong. Heavy bleaching may turn white cottons yellow reducing its strength.
5.2.4. Effect of Solvents:
Cotton is soluble in cuprammonium hydroxide and cupriethylenediamine. For rest of the solvents cotton exhibits resistance.
5.3. MISCELLANEOUS PROPERTIES:
5.3.1. Effect of Sunlight:
Prolonged exposure to sunlight disintegrates cotton by turning it to yellow. The presence of dyes and other finishing agents and pollutants may also augment the deterioration.
5.3.2. Effect of Mildew:
Cotton is affected by bacteria and fungi such as mildew. Cottons can be boiled safely to remove microorganisms. Storing away from moisture prevents from developing mildew black spots on fabric.
5.3.3. Effect of Moths:
Moths and beetles will not affect cotton but it is damaged by silver fish which eats cotton cellulose.
5.3.4. Effect of Perspiration:
Cotton is not affected by perspiration. However, the dyes used may not be fast.
6. FINISHES GIVEN TO COTTON:
Cotton, being in grey, may not be a fabric opted by everybody. There is large scope for finishing cotton to produce qualities in which cotton lacks.
- Preparatory finishes- Scouring, bleaching and singeing
- Routine finishes- Calendaring, tentering.
- Special purpose finishes-
- Sanforization – compressive shrinkage for maximum preshrinking.
- Mercerization – to improve strength, luster, absorbency and affinity for dyes.
- Wash n wear – for no ironing.
- Wrinkle resistance – free from wrinkles, for retention of appearance.
- Water repellency – for resistance to water and rain
- Stiffening- for building body and to provide smoothness
- Schrenerising – for producing luster on the surface of starched products
- Parchmentization- to impart transparency and permanent stiffness to cotton fabrics.
7. CONSUMER PREFERENCE:
Cotton is regarded as ‘king of fibres’ due to the following reasons:
- Cotton is a highly versatile It can be made into different fabrics starting from very light to heavy fabrics. Use of different methods of fabric construction has increased its versatile nature. Cotton fiber can be spun alone or it can be blended with other textile fibers such as linen, wool, silk, viscose rayon, polyester, and nylon. It serves the purpose of clothing or apparel, home furnishings and industrial fabrics by giving the comfort, durability, fashion and ease for care etc. The greatest amount of cotton is used for apparel purpose. As all cotton fabrics are used for comfort, and its appearance. Cotton blended with polyester is durable press fabric.
- It can be blended with polyester blended fabrics with specific features.
- It is highly comfortable for tropical climate of India. It provides coolness to the wearer by absorbing the sweat due to its hygroscopic nature. Its absorbency combined with good heat conductivity makes it highly suitable for summer wear.
- Generally cotton is durable and it can be made highly durable by carefully choosing the yarn twist and fabric construction and the finish. Knitted cottons form the best material for hosiery.
- Cotton’s ability to wash well is a boon to consumers. As cotton increases its strength when wet, it leaves the consumer with liberty to use friction for washing. It can withstand boiling during sterilization to make it suitable for hospital wear.
- Cotton fabric is considered as ‘ever green fabric’ by fashion goers. It can be styled to suit all fashion designs and thus it is on hot fashion.
- Industrial uses of cotton include abrasives, book bindings, luggage and handbags, shoes and slippers, tobacco cloth, woven wiping clothes, and wall covering fabrics.
Cotton Mark

REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi.Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- Wynne A (1997) Textiles The Motivate Series, Macmillan
- https://www.textileschool.com/129/cotton-fibers-the-king-of-fibers/
- https://journals.sagepub.com/doi/abs/10.1177/004051754801800201?journalCode=trjc
- https://www.textilemates.com/cotton-history-production-processing/
ASSIGNMENTS
- Identify textile products made of cotton fibers
- What are the finishes given to cotton textiles?
- Why cotton is called as ‘King of Fibers’?
- What types of clothes generally made of cotton that provide comfort to the wearer? List them
LINEN FIBER
1. INTRODUCTION:
Flax is a bast fibre, obtained from the plant, Linum usitatissimum. It is one of the oldest fibre known to mankind as early as 10000 to 8000 BC.
Flax is basically made of cellulose substance. Linen yarn is made from fibers removed from the stem of the slender flax. These fibers held together under the stem bark principally by a gummy substance (pectin).
2. HISTORY: Flax is one of the oldest textile fibers. Fragments of linen fabric have been found in pre historic lake dwellings in Switzerland, linen mummy wraps more than 3000 years old have been found in Egyptian tombs. There is evidence that flax was used as textile in 6000 & 4000 B.C. in the Middle East and Egypt. Until 18th century, flax was the major textile fibre, which was replaced with cotton, in the 19th and 20th century. Today flax is a prestige fiber as a result of its limited production and relatively high cost.
The unique and desirable characteristic of flax are its body, strength, durability, low pilling, and linting tendencies, pleasant hand, thick and thin texture.
Most flax is produced in Western Europe, in Belgium, France, Italy, Ireland, the United Kingdom, Germany, heathlands, Switzerland. Flax is also produced in Russia, Belarus and New Zealand
 3. STRUCTURE: The primary fiber of flax averages 5.0 to 21.5 inches in length and 12 to 16 micro meters in diameter. Flax fibers can be identified microscopically by crosswise markings called nodes or joints that contribute to its flexibility. The nodes may appear to be slightly swollen and resemble the joints in a stalk of corn or bamboo. In cross section, the fibre appears polygonal.
3. STRUCTURE: The primary fiber of flax averages 5.0 to 21.5 inches in length and 12 to 16 micro meters in diameter. Flax fibers can be identified microscopically by crosswise markings called nodes or joints that contribute to its flexibility. The nodes may appear to be slightly swollen and resemble the joints in a stalk of corn or bamboo. In cross section, the fibre appears polygonal.
Flax fibers are slightly greyish when due retted and more yellow when water retted. Short flax fibers are called tow, the long, combed; better quality fibers are called line. The line fibers are readied for wet spinning into yarn. The tow fibers must be carded before dry spinning into yarns for heavier fabrics for furnishings.
Flax fibre has a longer polymer and high degree of polymerization and greater orientation and crystalline area.
4. CHEMICAL COMPOSITION OF FLAX
|
Component |
% |
Contributing Property |
|
| Cellulose | 85-87% | ||
| Lignin | 2.5-4 % | Makes the cellulose rigid, is seen in amorphous areas; its presence has an impact on tenacity and resilience | If not removed properly it affects the hand and flexibility of the fibre |
| Pectin and Hemicelluloses |
19 % | Binds the fibre into bundles, determines lustre and hand of the fibre | If not removed properly it affects the fineness and spinning ability of the fibre |
5. MANUFACTURING PROCESSES
Fibre extraction of flax is similar to jute. During harvesting, the flax is pulled, not cut. Both biological and chemical retting can be done to the stalks. Biological retting produces better quality of fibres and is more economical. Cottonization of flax is a chemical retting. The steps in fibre extraction are as follows:
5.1 RETTING: Dew retting or water retting is done. Enzymes may also be used for retting, which act on the pectin.
5.2 BREAKING: The stalk becomes partially separated from the fiber when the wet plants are placed in the fields to dry. When the decomposed woody tissue is dried it is crushed by being passed through fluted iron rollers. This breaking operation reduces the stalk to small pieces of bark called shives.
5.3 SCUTCHING: It separates the bast from the woody core. The scutching machine removes the broken shives by means of rotating wooden paddles, thus finally releasing the flax fiber from stalk. This operation can be done by hand by beating the stalks with a blunt flat wooden or metal beater.
5.4 HACKLING (COMBING): This process prepares the fibres for spinning. The simple combing process known as hackling. It straightens the flax fibers separates the short from the long staple, and leaves the longer fibers in the parallel formation. For very fine linen, a hackling is done by hand. A faster and more efficient combing, hackling is done by machine.
This process produces
- Long fibre bundles called line
- Short tangled fibre called tow
5.5 SPINNING: The short staple flax fibers, called tow, are used for the spinning of irregular linen yarns. Tow is put through a carding operation, similar to the carding of cotton staple, which straightens the fibers and forms them into a silver ready for spinning into yarn. The long staple fibers are used for fine linens. These are called line, sometimes dressed flax. Line fibers are from 12 to 20 inches in length. They are put through machines, called spreaders, which combine fibers of same length, laying them parallel so that the ends overlap. The silver thus formed passes through sets of rollers making a rove for the final spinning process which inserts the final twist. Although flax is one of the strongest fibers, it is inelastic and requires a carefully controlled, warm, moist atmosphere for both methods of spinning.
5.6 DRY SPINNING: Dry spinning does not use moisture. It produces rough, uneven yarns, which are not especially strong. These yarns are used for making coarse, heavy and inexpensive linen fabrics.
5.7 WET SPINNING: This method requires a temperature of 120 Degree Fahrenheit, which is conductive to the production of soft ,even yarns .By passing the roving through hot water, the gummy substance on the fiber is dissolved, permitting drawing out the roving into the fine yarn of high yarn count.
6. PROPERTIES OF FLAX
6.1 Strength: Flax shows increased strength in wet condition, like cotton.
6.2 Tenacity: 4.8 to 6 g/denier
6.3 Specific gravity: 1.54
6.5 Elongation: low
6.6 Elastic recovery: 65% recovery at 2% elongation. Flax is a stiff fibre.
6.7 Drapability: Linen has more body than cotton and drapes better.
6.8 Heat conductivity: Linen has most suitable for summer apparel, as it allows the heat of the body to escape.
6.9 Moisture regain: 12% more absorbent than cotton
6.10 Absorbency: It absorbs moisture and dries more quickly. It is therefore excellent for handkerchiefs and towels.
6.11 Cleanliness and wash ability: Linen launders well and gives up stains readily, its softness is enhanced by repeated washing.
6.12 Reaction to bleaches: Linen does not stain readily as cotton, but it is also more difficult to bleach. Like cotton, it is weekend by sodium hypochlorite bleaches, sodium perborate bleaches are effective and safer.
6.13 Shrinkage: Linen does not shrink but preshrink age finishing is desirable.
6.14 Effect of heat: Linen scorches and flames in a manner similar to cotton.
6.16 Effect of light: Linen is more resistant to light than cotton, but it will gradually deteriorate from protracted exposure.
6.17 Resistance to mildew: Like cotton, linen is vulnerable to mildew.
6.18 Resistance to insects: Like cotton, linen is not damaged by moths. Damage by other insects is uncommon.
6.19 Reaction to alkalies: Linen like cotton is highly resistant to alkalies. Linen may also be mercerized.
6.20 Reaction to acids: Linen is damaged by hot dilute acids and cold concentrated acids but not by cold dilute acids.
6.21 Affinity for dyes: linen does have good affinity for dyes. However, it is possible to obtain dyed linen that has good colorfastness.
6.22 Resistance to perspiration: Acid perspiration will deteriorate linen. Alkali perspiration will not cause deterioration. But discoloration may occur.
6.23 Resistance to sunlight: More sunlight resistant than cotton
7. FINISHING PROCESSES: Linen is commonly treated by bleaching.
7.1 BLEACHING: Two methods are used grass bleaching and chemical bleaching. Grass bleaching the finest results. Although chemical bleaching is chiefly used , it may adversely effect the durability of the finished fabric owning to the weakening effects of the chemicals.
7.2 BEETLING: For flexibility and thickness.
7.3 CALENDERING: For luster and smoothness.
7.4 MERCERIZING: For luster
7.5 SIZING: for added body
7.6 WRINKLE RESISTANCE: For resilience and easier care.
8. USES OF LINEN
Short coarse fibres are used in making pulp for paper, cigarette paper, packaging, laminates, coatings, particle boards, non wovens for geo textiles and chemo textiles.
9. MEASURE
The standard measure of flax yarn is cut .If one pound of flax is drawn out to make 300 yards, the yarn is known as Ne 1. When drawn out to make twice 300 yards, it is labelled Ne 2 .The higher the yarn count, the finer the yarn.
10. CARE OF LINEN: Linen fabrics can be dry cleaned, machine washed or bleached with chlorine bleaches. As linen fabrics have low resiliency, they tend to crease more and require pressing. Linen is resistant to alkalis, organic solvents and high temperature.
11. CONSUMER PREFERENCE: Linen has long been used for a wide variety of apparel and home furnishings. In fact, the oft used term of linens which refers to such home furnishings as sheet, pillowcases, towels, table cloths. Linen is now used to a limited extent. Linen apparel includes items for warm weather use, high fashion, casual and professional wear. Industrial products include luggage, bags, purses and sewing threads. Linen fabrics are used in upholstery and window treatment, because of their durability, interesting and soil hiding textures and versatility in fabrication and design.
REFERENCES
- Textiles, 10th addition, Sara J. Kadolph, Published by, Dorling Kindersley India Pvt. Ltd.
- Textiles – Fiber to Fabric, 6th addition, Bernard P. Corbman.
- Handbook of Natural Fibres: Types, Properties and Factors Affecting Breeding and cultivation. Vol. I, Edited by Ryszard M Kozłowski, Woodhead Publishing, 2012
ASSIGNMENT
- List the products made of Linen
- List the reasons why linen is more absorbent that cotton
- Collect the pictures regarding the linen products
JUTE FIBRE
1. INTRODUCTION
Jute is extracted from the bark of the white jute plant, Corchorus capsularis and to a lesser extent from tossa jute (C. olitorius). It flourishes in tropical lowland areas with humidity of 60% to 90%. A hectare of jute plants consumes about 15 tonnes of carbon dioxide and releases 11 tonnes of oxygen. Yields are about 2 tonnes of dry jute fibre per hectare.
Jute is a natural fiber popularly known as the golden fiber. It is one of the cheapest and the strongest of all natural fibers and considered as fiber of the future. Jute is second only to cotton in world’s production of textile fibers. India, Bangladesh, China and Thailand are the leading producers of Jute. It is also produced in southwest Asia and Brazil. The jute fiber is also known as Pat, kosta, Nalita, Bimli or Mesta (kenaf).
Kenaf known as Mesta or Ambari (species Hibiscus Cannabinus) is also considered as a variety of Jute. It is cultivated in Indian subcontinent, Thailand, China and Africa. The two main types of jute, white jute (Corchorus Capsularies)and dark jute or tossa (Corchorus Olitorius) are grown in India, Bangladesh, Thailand, China, south Asian countries and Brazil.
India is the largest producer of jute goods in the world, while Bangladesh is the largest cultivator of raw jute. The cultivation of Jute in India is mainly confined to the eastern region states – West Bengal, Bihar, Assam, Tripura, Meghalaya, Orrissa and Uttar Pradesh. Nearly 50 percent of total raw jute production in India alone figures in West Bengal.
2. CULTIVATION OF JUTE
Jute requires a warm and humid climate with temperature between 24° C to 37° C. Constant rain or water-logging is harmful. The best soil for jute is the gray alluvial soil of good depth, receiving salt from annual floods. Jute is harvested between 120 days to 150 days from sowing when the flowers have shed. Early harvesting gives good healthy fibers. The harvested plants are left in field for 3 days for the leaves to shed. The stems are then made up into bundles for steeping in water. Steeping is carried out immediately after harvest.
3. PROCESSING OF JUTE
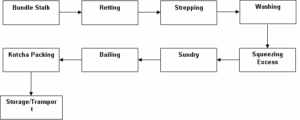
 3.1 Retting: Retting is the process of extracting fiber from stem of the plants. The various ways of Retting are:
3.1 Retting: Retting is the process of extracting fiber from stem of the plants. The various ways of Retting are:
Mechanical retting (hammering), chemical retting (boiling & applying chemicals), steam/vapor/dew retting, and water or microbial retting. Among them, the water or microbial retting is a century old but the most popular process in extracting fine fibers. However, selection of these retting processes depends on the availability of water and the cost of retting process.
To extract fibers from jute plant, a small stalk is harvested for pre-retting. Usually, this small stalk is brought before 2 weeks of harvesting time. If the fiber can easily be removed from the Jute core, then the crop is ready for harvesting.
After harvesting, the jute stalks are tied into bundles and submerged in running water. The stalk stays submerged in water for around 20 days. However, the retting process may require less time if the quality of the jute is better. In most cases, the fiber extraction process of fibers in water retting is done by the farmers standing under water.
When the jute stalk is well retted, the stalk is grabbed in bundles and hit with a long wooden hammer to make the fiber loose from the jute. After losing the fiber, the fiber is washed with water and squeezed till the last drop of water. The extracted fibers is further washed with fresh water and allowed to dry on bamboo poles. Finally, they are tied into small bundles to be sold into the primary market.
4. EXTRACTION OF FIBRE:
The jute plant’s fibers lie beneath the bark and surround the woody central part of the stem. To extract the fibers from the stem, the process is carried out in the following stages:
5. JUTE FIBRE MORPHOLOGY
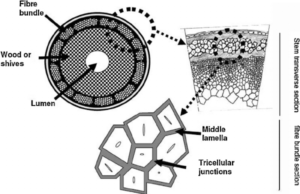
Jute is an agricultural product and chemically known as ligno-cellulosic fiber. The fibres are arranged in the bast or phloem region of the jute plant consisting of pyramidal wedges, fibre bundles in each wedge are further arranged in large number (8 to 12) layers. The ultimate cells of individual fibres are formed by the alpha-cellulose where as the presence of hemi-cellulose and lignin cements the ultimate fibres. As a result jute fibres form a mesh or network in which the individual fibres or strands have no identity.
6. FIBRE COMPOSITION:
Jute is mainly composed of polysaccharides and lignin but it also contains smaller amount of fats and waxes, pectin, nitrogenous, coloring and inorganic matters. The polysaccharides or glucose units are of two types such as alpha-cellulose (C6H10O6)n and hemi-cellulose.
|
Chemical composition of jute fibre |
|
|
Constituents |
% |
| Cellulose | 60 – 62 |
| Hemi Cellulose | 22 – 24 |
| Lignin | 12 – 14 |
| Others | 1 – 2 |
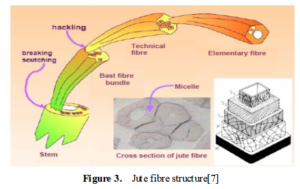 7. STRUCTURE OF JUTE:
7. STRUCTURE OF JUTE:
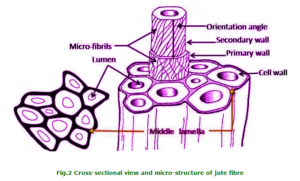
Jute is multicelled in structure (Fig. 3). The cell wall of a fibre is made up of a number of layers: the primary wall and the secondary wall (S), which again is made up of the three layers (S1, S2 and S3). As in all lignocellulosic fibres, these layers mainly contain cellulose, hemicellulose and lignin in varying amounts. The individual fibres are bonded together by a lignin-rich region known as the middle lamella. Cellulose attains highest concentration in the S2 layer (about 50%) and lignin is most concentrated in the middle lamella (about 90%) which, in principle, is free of cellulose. The S2 layer is usually by far the thickest layer and dominates the properties of the fibres. Cellulose, a primary component of the fibre, is a linear condensation polymer consisting of Danhydro- glucopyranose units joined together by ß-1, 4-glucosidic bonds. The long chains of cellulose are linked together in bundles called micro-fibrils (Fig. 3).
Jute is long, soft and shiny, with a length of 1 to 4 m and a diameter of from 17 to 20 microns. It is one of nature’s strongest vegetable fibres and ranks second only to cotton in terms of production quantity. Jute has high insulating and anti-static properties, moderate moisture regain and low thermal conductivity.
8. PROPERTIES OF JUTE FIBRE
| Property |
Jute |
Cotton |
| Ultimate cell length, L (mm) |
0.8 to 6.0 |
15 to 35 |
| Ultimate cell breadth, B (mm) |
10 to 25μm |
4 to 6μm |
| Length / Breadth (L/B) Ratio |
110 |
25,000 |
| Fineness (Denier) |
15 to 35 |
1.5 to 3.0 |
| Tenacity (gm/denier) |
3 to 5 |
2.5 to 4 |
| Elongation at break (%) |
1.0 to 1.8 |
6.5 to 8 |
| Density (gm/cc) |
1.46 |
1.56 |
| Degree of crystallinity (X-ray) |
55 to 60 % |
— |
| Angle of orientation (X-ray) |
7 to 90 |
— |
| Initial modulus |
17 to 30 N/tex |
|
| Flexural rigidity (dynes.cm) |
3.0 to 5.0 |
0.3 to 1.0 |
| Moisture Regain (%) at 65% R.H. |
12.5 |
8.5 |
| Moisture Regain (%) at 100% R.H. |
36 |
24 |
| Diameter swelling (%) at 100 % RH |
20 to 22 |
20 to 22 |
9. PHYSICAL PROPERTIES
9.1 Strength: Jute is a strong but low extensible fibre mainly due to composite like structure with highly oriented long chain molecules. Tossa jute is stronger than white jute.
9.2 Elasticity: Low
9.3 Resiliency: Low
9.4 Abrasion resistance: Very low
9.5 Moisture absorbency: Due to presence of numerous polar –OH groups, jute fiber shows good moisture absorption capacity.
9.6 Moisture Regain: Moisture regain value may be upto 36% at 100% relative humidity which is much higher than cotton. Since long chain molecules are almost aligned along the fibre axis, the swelling of jute fiber is found to be much more laterally than longitudinally. The diameter wise and cross sectional wise swelling of jute in water is about 20% and 44% respectively which are higher than cotton but the longitudinal swelling is only 0.4%. Water holding capacity of jute is about 500%.
10. THERMAL PROPERTIES: On heating to high temperature, jute fibre chars and burns without melting like cotton. Ignition temperature of jute is about 1930C.The high specific heat value (1360 J/kg/K) results good thermal insulation of jute.
11. CHEMICAL PROPERTIES:
11.1. Effect of alkalies: Jute fibres have poor resistance to alkali due to presence of hemicelluloses. Alkali treatment extracts out the hemicelluloses from the fibre structure making it weak. Treatment with 18 % caustic soda jute becomes slightly weaker but highly soft and crimpy due to irregular swelling. The process is popularly known as woollenisation.
11.2. Effect of acids: Jute fibres are weakened and destroyed by acids. The cellulose chains disintegrate due to hydrolysis in presence of acids. The mineral or inorganic acids are more effective than organic acids.
11.3. Effect of bleaches: The bleaching agents remove the natural colour from jute to make it white but at the same time partly remove the lignin and make the jute weaker and finer.
12. USES OF JUTE:
Jute is the second most important vegetable fiber after cotton not only for cultivation, but also for various uses. Jute is used to make sacks and coarse clothes and also being used for wrapping bales of raw cotton. Jute yarns are woven into fine fabrics to use as curtains, chair coverings, cheap quality rugs, hessian clothes, backing of linoleum and carpets. Jute was mainly used for making sacks but now things have changed and many other ways of utilizing jute has come up.
Jute began to lose its popularity with the advent of synthetic materials as the latter was more economical. But slowly people began to realize the negative impact of synthetic materials on the environment. Jute scores over synthetic materials largely due to its bio-degradable nature. Examples of such uses include containers for saplings, which can be planted directly with the container without disturbing the roots, and land restoration where jute cloth prevents soil erosion.
The fibers are used to make ropes. Jute rope has long been popular in Japan for use in bondage. Jute butts, the coarse ends of the plants, are used to make cheap clothes. It also helps in addressing the problem of deforestation as it also makes. Jute has a long history of use in the sackings, carpets, wrapping fabrics (cotton bale), and construction fabric manufacturing industry.
Jute can be used to create a number of fabrics such as Hessian cloth, sacking, scrim, carpet backing cloth (CBC), and canvas. Hessian, lighter than sacking, is used for bags, wrappers, wall-coverings, upholstery, and home furnishings. Sacking, a fabric made of heavy jute fibers, has its use in the name. CBC made of jute comes in two types. Primary CBC provides a tufting surface, while secondary CBC is bonded onto the primary backing for an overlay.
Diversified jute products are becoming more and more important to the consumer today. Among these are gift articles, handicrafts, wall hangings, shopping & carry bags, floor coverings, home textiles, high performance technical textiles, Geotextiles, composites etc.
13. FEATURES OF JUTE:
- Jute fiber is 100% bio-degradable and recyclable and thus environmentally friendly.
- It takes very short time to grow (4-6 months) which can be useful in growing other food crops.
- It is a natural fiber with golden and silky shine and hence called The Golden Fiber.
- It is the cheapest vegetable fiber procured from the bast or skin of the plant’s stem.
- It is the second most important vegetable fiber after cotton, in terms of usage, global consumption, production, and availability.
- It has high tensile strength, low extensibility, and ensures better breathability of fabrics. Therefore, jute is very suitable in agricultural commodity bulk packaging.
- It helps to make best quality industrial yarn, fabric, net, and sacks. It is one of the most versatile natural fibers that have been used in raw materials for packaging, textiles and non-textile construction, and agricultural sectors. Bulking of yarn results in a reduced breaking tenacity and an increased breaking extensibility when blended as a ternary blend.
- The best source of jute in the world is the Bengal Delta Plain in the Ganges Delta, most of which is occupied by Bangladesh.
- Advantages of jute include good insulating and antistatic properties, as well as having low thermal conductivity and moderate moisture regain. Other advantages of jute include acoustic insulating properties and manufacture with no skin irritations.
- Jute has the ability to be blended with other fibers, both synthetic and natural, and accepts cellulosic dye classes such as natural, basic, vat, sulfur, reactive, and pigment dyes. The resulting jute/cotton yarns will produce fabrics with a reduced cost of wet processing treatments.
- Jute can also be blended with wool. By treating jute with caustic soda, crimp, softness, pliability, and appearance of it are improved, aiding in its ability to be spun with wool. Liquid ammonia has a similar effect on jute, as well as the added characteristic of improving flame resistance when treated with flame proofing agents.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- http://www.jute.com/green-jute/agriculture-raw-jute
- http://www.worldjute.com/about_jute/abj_intro.html
ASSIGNMENT:
- Collect any three products/ jute fibers from different products used at home
- Collect pictures of apparel products made of jute fibers or its blends
- List the uses of jute in various products around you
RAMIE FIBER
1. INTRODUCTION
Ramie belongs to the genus Boehmeria, commonly known as china grass, white ramie, green ramie and rhea. It is perennial plant, a member of the order Urticaceae or nettle which can be harvested six times a year. It is one of the oldest vegetable fibers and has been used for thousands of years. It produces a large number of un branched stems from underground rhizomes and has a crop life from 6 to 20 years. Chemical treatment is given to fibers for the removal of gums and pectins found in the bark.
Same process is used to transform ramie fibers into fabric as used for manufacturing linen from flax. The true ramie or China Grass or white ramie is cultivated in China. It has large heart shaped, broad leaves covered with white hair responsible for silvery appearance. Green ramie or rhea has smaller leaves than white ramie and is better suited to tropical climates, cultivated in peninsula. Normally ramie plant grows 1-2.5 m high. The fibers are located in the cortex layer of the stem underneath the thin bark. Leading producers of ramie are China, Taiwan, Korea, the Philippines and Brazil.
Ramie in China is usually harvested by hand as the canes mature. Uneven strands of ramie, makes harvesting a difficult task. Pounding and scraping are necessary processes to separate the fibres. After separation of fibers from woody matter and soft tissues, fibres remain in ribbon-like strips as they are held together by gums and pectin. The fibres are degummed by boiling or acid. Degumming weakens the fibre. Machines are not yet capable of performing all the steps necessary which is why ramie is not widely used.
It is often blended with cotton to make woven and knit fabrics. It is used in clothing, tablecloths, napkins and handkerchiefs. Apart from textile industry, ramie is used in fish nets, canvas, upholstery fabrics, straw hats and fire hoses.
2. PROCESSING OF RAMIE
Ramie’s fibers are present in the bark of the stalk. The stems are soaked in water for a few hours. The inner fiber is stripped away from the skin using a blunt knife. After drying the fibre in the shade, it is then split into narrow strips.
2.1 Stripping: Stripping is a process in which removal of all the phloem and some outer bark is carried out. The resulting strips are often referred to as China grass. Hand stripping is generally carried out on fresh stems and is said to yield better quality fiber than that produced by decorticating dry stems.
2.2 Ribboning: Process involving removal of the outer bark/epidermis and the bast from the woody core of the stem is referred as ribboning. Ribbons contain more of the outer parts of the stem than strips. The stems are usually fed between longitudinally fluted rollers for the removal of ribbons which crush the woody core and knock any wood fragments out of the bast. However, ribbons may also be obtained by another process using a modified decorticator in which the core is removed from the stem by the action of a moving drum.
2.3 Decortication: Mechanical decortication is most effectively carried out on either fresh green stems or dry stems. Dry decortication is a quicker process and has the advantage of not being restricted to the harvesting season. However, decortication of fresh stems is said to produce a better quality fiber. Mechanical decorticators built on the principle of subjecting the stem to a succession of blows to break up the woody core. The principle of successive slow blows treats the fibers more mildly and is congenial for the mechanical properties of the fiber. High velocity blades disintegrate stems which are fed into the drum resulting in fiber separation. The blades subsequently scrape away the epidermal, woody tissues from the fiber.
2.4 Washing and drying: The water soluble gums need to be removed after decortications and is done by washing. This also decreases transport costs to the degumming site. The extracted fibrous material, after washing, should be immediately dried or degummed to prevent the development of mildew. Degumming of fresh ribbons prevents loss in fiber value caused by mildew which develops in slowly or incompletely dried fiber.
3. CHEMICAL COMPOSITION OF RAMIE
| Table 2: Chemical composition of ramie fibre | ||||||||||
|
4. PROPERTIES
4.1. PHYSICAL PROPERTIES
4.1.1. Structure: Ramie fibres exhibit the best mechanical properties in the group of bast fibres (45-88 cN/tex). The fibre length ranges between 120-150mm and fibre diameter is 40-60 μm. The main drawback of ramie is its low elasticity of 3-7% which means that it is stiff and brittle. Cross sectional view of fibers are oval to cylindrical in shape. Rough surface of fiber are characterized by small ridges, striations, and deep fissures. It can be easily identified by its coarse, thick cell wall, lack of twist and surface characteristics. The fiber of ramie is very fine like silk. It is naturally white in colour and lustrous. The physical form of the cellulose is rigid and crystalline, but porous sieve-like makes it with even better absorbency than other cellulose fibres. In addition, ramie is softer and has better dyeability. Ramie’s fibres are found in the bark of the stalk. The process of transforming ramie fibre into fabric is similar to producing linen from flax.

Fig 1: (a) Longitudinal view (b) Cross-section through ramie fibres with lumen (cavity in fibre)
4.1.2 Properties of Ramie Fiber
- Ramie is one of the strongest natural fibers.
- It is better strength when wet.
- It is similar to linen.
- It is not durable as other fibers, and so is usually used as a blend with other fibers such as cotton or wool.
- It is known especially for its ability to hold shape, reduce wrinkling, and introduce a silky lustre to the fabric appearance
5. ADVANTAGES OF RAMIE
- Resistant to bacteria, mildew and insect attack.
- Extremely absorbent.
- Dyes fairly easy.
- Increases in strength when wet.
- Withstands high water temperatures during laundering.
- Smooth lustrous appearance improves with washing.
- Keeps its shape and does not shrink.
- Can be bleached.
6. DISADVANTAGES OF RAMIE
- Low in elasticity.
- Lacks resiliency.
- Low abrasion resistance.
- Wrinkles easily.
- Stiff and brittle.
7. USES OF RAMIE: Ramie has limited acceptance for textile use. It is used to make industrial sewing thread, packing materials, fishing nets, and filter cloths. Fabric of ramie is used for household furnishings and clothing. It is blended with other textile fibers for example wool, as result shrinkage is reported to be greatly reduced when compared with pure wool. Shorter fibers and waste are used in paper manufacturing.
8. CARE OF RAMIE
Ramie-blend fabrics can be laundered or dry-cleaned depending on the dyes, finishes and garment design. The care label will state the preferred method. The dry-cleaning method helps preserve the beauty of woven ramie items and gives best colour and shape retention and a wrinkle free appearance. With caution, white ramie fabrics may be bleached with chlorine-type bleaches. Ramie fabrics withstand ironing temperatures up to 400 to 450 degrees F or the cotton setting on an iron.
When storing ramie or ramie blends, lay them flat. Ramie fibres are brittle and tend to break. Avoid folding the garment or pressing sharp creases in woven fabrics.
REFERENCES
- Textiles, 10th addition, Sara J. Kadolph, Published by, Dorling Kindersley India Pvt. Ltd.
- Textiles – Fiber to Fabric, 6th addition, Bernard P. Corbman.
- Handbook of Natural Fibres: Types, Properties and Factors Affecting Breeding and cultivation. Vol. I, Edited by Ryszard M Kozłowski, Woodhead Publishing, 2012
- http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1022&context=tsaconf
- http://info.fabrics.net/fabric-facts/hemp-ramie-and-jute/
ASSIGNMENT
- List the products made of Ramie
- Collect the pictures regarding the processing of Ramie
HEMP FIBER
1. INTRODUCTION
Hemp belongs to the Mulberry family (Moracea) and cultivated hemp varieties belong to the Cannabis sativa species. These hemp varieties can be very different in height and leafage. Hemp is one of the fibres which are usually named after their country of origin – thus Italian, Turkish, Chinese, Indian hemp varieties are available. It is an annual plant, grows in season from the middle of April to the middle of September. The plant can be monoecious or dioecious. The bast layer (phloem) of the stalk contains more important component of textile fibres.
2. PHYSICAL AND CHEMICAL PROPERTIES
The fibre bundles are in several layers within the hemp stalks. A bundle consists of several fibres and bundles are connected by unit cells. The bundles in the inner layers are usually shorter and finer than those of the outer layers. The shape of unit cells ranges from triangular to heptagonal with rounded corners and a large pith. Unit cells are connected by lignified pectins and the basis of hemp processing lies in loosening and dissolving this bond.
3. MECHANICAL PROPERTIES
Its flexibility depends on the fineness of the bundle. The longer bundles require less twist during spinning. The elongation of the bundles is low and their flexibility is high and this can cause problems during spinning. Blending flax with hemp improves both the elongation and the flexibility of the yarns, which is low in 100% hemp yarns. However, these blends also decrease the strength of the yarn.
4. PRIMARY PROCESSING OF HEMP STALKS, FIBRE SEPARATION
Primary processing on hemp is done to split the flexible bast fibres from the ligneous hurds as gently as possible or when necessary, using more powerful biological and mechanical processes. Naturally the application of these processes is determined by several parameters. The quality of the hemp stalks; colour, length, diameter, etc., fibre yield and ratio of long and short fibres, and quality of the fibres like colour, strength, fineness, cleanliness, etc are all influenced by the process chosen. This is similar to other bast fibres as well.
Table 1 Yields during primary processing
| Before retting | Raw hemp stalks | 100% |
| After retting | Retted hemp stalks | 90% |
| Mechanical processing | Hurds Scotched hemp Hemp tow Waste |
61% 10% 12% 7% |
Different technical and mechanical factors are taken into account as to which process should be chosen and which particular technology is chosen depends on the quality of the stalks. They are classified as follows:
- a) Water retted fibres used for finer yarns, medium count and thicker yarns
- b) Unretted fibres mechanically separated and used for blending or spinning coarse yarns.
Fibre separation may be done by using biological, mechanical or chemical methods or more usually a combination of retting, breaking and scutching.
4.1 RETTING
Pectic substances bind the fibres to constituents of the hemp stalk. This binding material needs to be removed during retting process. Retting is followed by mechanical separation of the fibre. During retting the moisture content of the stalks is increased. Moisture is very important in the process as it encourages growth of certain bacteria and/or fungi which selectively attack and remove the pectic contents. There are two methods of retting, ground and water retting.
4.2 Ground retting
In ground retting the stalks are laid on the ground after they are harvested. The combined action of dew and showers of rain provide the necessary conditions for the development of the micro-organisms on the stalks. Ground retting is highly dependent on abundant rainfall and thus is restricted in areas which receive heavy rainfall.
4.3 Water retting
In the second type of retting, the necessary increase in moisture content is obtained by steeping bunches of stalks in tanks containing water. The stalks, which are quite heavy, are lifted into the tanks by mobile cranes and held beneath the surface of the water by wooden or iron frames which cover the tanks. Water can be used from various sources like flowing rivers or from bore-wells. It is important to note that higher the temperature results in shorter retting period.
When retting is sufficiently advanced the stalks are gathered and stooked in the open fields so that they can dry. This stops the retting process and the stalks are then stacked in large ricks until they are required for further processing. These are thatched with bundles of hemp stalks to protect them from the weather. The advantage of ground retting over water retting is that it is more economical. The disadvantage is that the process is difficult to control and depends entirely on favourable meteorological conditions
4.5 Mechanical processing
The purpose of mechanical fiber separation, or decortication, is to separate the flexible fibres from the stiff and more brittle ligneous woody parts (called hurds) of the stalks.
4.6 Breaking
The stalks are delivered to the primary processing plant in bundles or `sheaves’. The first step is to open these sheaves. Subsequently the stalks are fed into vertical breaking rolls or placed on a conveyor which feeds them through horizontal parallel breaking rolls. The first breaking operation consists of splitting the stalks down their lengths. The actual breaking process is done by the action of pairs of smooth and ribbed breaking rolls on the stalks. After separation these separate from the fibre and fall through or out at the end of the machine before the start of the next operation.
Scutching
The principal purpose of scutching is to remove the hurds which still adhere to the fibres bundles. Scutching further softens the fiber bundles and removes lignin from the fibers. Earlier, scutching used to be done by hand and this produced good-quality fiber. However, productivity was low and the process was therefore uneconomical; in due course it was replaced by mechanical (turbine) scutching.
In mechanical processing, turbine scutching is popular. In this process, the broken stalks are fed into grippers that present the stalks, held vertically, between two scutching turbines. These turbines have around four blades which, as the turbines rotate in opposite directions, beat the stalks and thus remove the hurds. The effectiveness of scutching is controlled by varying the speed of rotation of the turbines. Turbine scutching produces fibers whose quality is the average of the batch being processed. It does not enable any selection of fibers of different quality.
Hemp tow processing
The preliminary mechanical processing of the dried stalks involves some fairly vigorous handling of the raw material, including breaking, scutching and the separation of the line fibers from the tow and other by-products. The weaker and shorter fibers break away and are removed together with the hurds, dust, soil, etc., by a pneumatic suction system placed below the scutching turbines.
The hemp tow is subsequently passed through a conveyer drier that can use either hot or cold air. After drying the tow is passed through various breaking and cleaning rollers and over further reciprocating screens, all aimed at cleaning the fibers and removing impurities but in addition, the high pressure breaker rollers further split the tow fibers. These are all high output processes.
5. APPLICATIONS OF HEMP
It is mainly used to make rope, canvas and paper. From long fibers fabric can be spun and woven to make crisp linen like fabric to use in clothing, home furnishing textiles. Due to its lower lignin content it is also used in paper industry. As reinforcing agent it is used to make molded thermoplastics in the automobile industry.
REFERENCES
- Textiles, 10th addition, Sara J. Kadolph, Published by, Dorling Kindersley India Pvt. Ltd.
- Textiles – Fiber to Fabric, 6th addition, Bernard P. Corbman.
- Handbook of Natural Fibres: Types, Properties and Factors Affecting Breeding and cultivation. Vol. I, Edited by Ryszard M Kozłowski, Woodhead Publishing, 2012
- https://www.youtube.com/watch?v=kauSxTJc3zs
- https://fashionunited.uk/news/business/sustainable-textile-innovations-hemp-fibres/2017071025112
ASSIGNMENT
- List the products made of Hemp
- Collect the pictures regarding the processing of Hemp
WOOL FIBRE
1. INTRODUCTION
Wool was the one of first fibers to be converted into fabric. It is obtained from the fleece or hair of the sheep or lamb of Angora or Cashmere goat. It is a staple fiber varies from 5- 45 cm, with natural color of white, ecru, grey, to brown and black. It is composed of protein and natural grease hence it gives the burning hair smell when introduced into the flame.
Merino sheep are known for providing the finest wool. There can be 60,000 wool follicles per square inch of the skin of merino sheep totaling approximately 100 million fibers in one merino fleece.
2. HISTORY OF WOOL
Originally, wool was borne on wild species of sheep as a short, fluffy undercoat concealed by hair. When primitive people killed wild sheep for food, they used the pelts as body coverings. The fluffy undercoat probably became matted by usage, thus giving early man the idea of felting it into a crude cloth. In first century A.D. itself man discovered that Merino sheep could be bred to improve the fleece, as the wool of wild sheep is coarse. The breeding of the animals and the production of the wool fiber into fabric are more costly processes consequently wool fabrics are more expensive.
3. WOOL PRODUCING COUNTRIES
Cold weather produces a hardier and heavier fiber. Excessive moisture dries out natural grease. Insufficient or poor food retards growth. Certain countries are suitable for large-scale sheep raising and consequently produce the greatest quantities of wool. The chief wool producing countries are Australia, the U.S.S.R., New Zealand, Argentina, South Africa, Uruguay, and the United States.
4. TYPES OF WOOL
Generally wool is graded based on
- Fibre fineness or diameter
- Length
- Age of the animal
- Location of the fibre
- Natural colour
- Sheep breed
- Conditions under which animal lived
An average of 8 pounds of wool is clipped from a sheep in a year and after cleaning and scouring it reduces 5 kgs of wool.
4.1 Wool products labeling act
The U.S. government passed the Wool Products Labeling Act in July 1941. This act was amended in 1980. The wool is variously called salvaged, reclaimed, reworked, or re manufactured, but it is best known in the textile industry as shoddy. Other definitions used for wool labeling are:
4.1.1 Wool: It must always mean new wool. New wool comes directly from a fleece. It has never been previously spun, woven, felted, or worn.
4.1.2 Virgin wool: It is now used by the textile industry to designate new wool from a sheep’s fleece, but the term is too all-inclusive to serve as criteria of quality i.e, low grade or high grade wool.
4.1.3.Pure wool: It the wool fabric made of 100 % wool fiber only.

4.1.4.Wool blend: It is the fabric containing both wool fibers and any other textile fibers.
4.1.5 Recycled Wool: According to the government classification, recycled wool is fiber that has been reclaimed and remanufactured from used or unused wool materials.
4.1.6 Lamb’s wool: It refers to the first fleece sheared from a lamb of six to eight months old. This fibre is fine and soft due to the age of the lamb and tapered ends of hair that are not shorn earlier. Lamb’s wool is generally used for children’s clothing.
4.1.7 Hogget wool: Refers to the fleece obtained from sheep of 12 to 14 months old that have not been shorn previously. The fiber is fine, soft, matured, strong, resilient.
4.1.8 Weather wool: Refers to any fleece clipped after the first shearing from matured animal. It lacks the softness of hogget wool and is soiled.
4.1.9 Pulled wool: Refers to the hair pulled from the sheep after slaughtering the animal for meat. This wool is considered as inferior wool as the animal raised for slaughtering will not have good quality wool and also the use of chemicals for pulling damages the roots.
4.1.10 Dead wool: Refers to wool that has been recovered from sheep that have died on the range or killed accidently. This grade is considered inferior.
4.1.11 Cotty wool: It is obtained from the sheep that are exposed to severe weather conditions or malnourished. The fiber is hard and brittle and resembles cotton, hence the name.
4.1.12 Taglocks: This refers to an inferior grade of wool fleece that has been torn, ragged or discoloured. Taglocks are marketed separately.
Irrespective of the breed of the sheep, superior wool is found to grow longer, finer and softer on sides and shoulder of the animal. Wool obtained from the head, chest, belly and shanks is considered little inferior and treated as a second fleece.
5. MANUFACTURING PROCESSES
5.1 Shearing:
Sheep are generally shorn of their fleeces in spring, but the time of shearing rises in different parts of the world. Machine clippers remove the fleece faster, closer than the hand clippers. Superior comes from the sides and shoulders, where it grows longer, finer, and softer, is treated as one fleece; wool from the chest, belly, and shanks is treated as a second fleece
5.2 Preparation:
An average about 8 pounds of fleece is made from one sheep. Then the fibers are packed in bags or bales. The raw wool or newly sheared fleece is called grease woolbecause it contains the natural oil of the sheep. When grease wool is washed, it loses from 20 to 80 percent of its original weight. The grease, known as yolk, is widely used in the pharmaceutical and cosmetic industries for lanolin compounds.
5.3 Sorting and Grading:
Wool sorting is done by skilled workers. Each grade is determined by type, length, fineness, elasticity, and strength. It is done by separating the fiber by touch and sight.
5.4 Scouring:
Washing of raw wool in an alkaline solution is known as scouring. The wool is treated with warm water, soap, and a mild solution of soda ash or other alkali to remove the dirt in the fibers.
If the raw wool is not sufficiently clear of vegetable, substance after scouring, it is put through the carbonizing bath.The fibers are then put through a dilute solution of sulfuric or hydrochloric acid, which destroys any vegetable. This process is known as carbonizing, and the resultant wool fibers are called extracts.
To remove the grease and dirt in the raw wool it is put through a series of naphtha baths followed by clear water to remove the naphtha. This is called as Naphthlation. It improves the dye uptake property of the wool.
5.5. Garnetting:
Recycled wool fibers are obtained by separately reducing the unused and used materials to a fibrous mass by a picking and shredding process called garnetting.
5.6 Drying:
Wool is not allowed to become absolutely dry. Usually, about 12 to 16 percent of the moisture is left in the wool to condition it for subsequent handling.
5.7 Oiling:
As wool is unmanageable after scouring, the fiber is usually treated with various oils, including animal, vegetable, and mineral, or a blend of these to keep it from becoming brittle and to lubricate it for the spinning operation.
5.8 Dyeing:
If the wool is to be dyed in the raw stock, it is dyed at this stage. Some wool fabrics are piece-dyed, some are yarn or skein dyed, and some are top-dyed.
5.9 Blending:
Wool of different grades or pure wool fibers and other textile fibers may be blended or mixed together at this point. All this information should present on the labels.
5.10 Carding:
The carding process introduces the classifications of woolen yarns and worsted yarns. It makes the fiber parallel and some amount of dirt is removed due to the straightening the fibers. Fibers used for the worsted yarn are more straightened than the the woolen yarns.
5.11 Gilling and Combing:
The carded wool, which is to be made into worsted yarn, is put through gilling and combing operations. The gilling process removes the shorter staple and straightens the fibers. This process is continued in the combing operation, which removes the shorter fibers of 1 to 4 inch lengths (combing noils) places the longer fibers (tops) as Parallel as possible, and further cleans the fibers by removing any remaining loose impurities.
5.12 Drawing:
Drawing is an advanced operation for worsted yarns which doubles and redoubles slivers of wool fibers. The process draws, drafts, twist and winds the stock, making the slivers more compact and thinning them into slubbers.
5.13 Roving:
This is the final stage before spinning. Roving is actually a light twisting operation to hold the thin slubbers intact.
5.14 Spinning:
In the spinning operation, the wool roving is drawn out and twisted into yarn. Woolen yarns are chiefly spun on the mule-spinning machine. Worsted yarns are spun on any kind of spinning machine -mule, ring, cap, or flyer.
The differences between woolen and worsted yarns are as follows:
| Woolen Yarn | Worsted Yarn |
| Short staple | Long staple |
| Carded only | Carded and combed |
| Slack twisted | Tightly twisted |
| Weaker | Stronger |
| Bulkier | Finer, smoother, even fibers |
| Softer | Harder |
5.15 Weaving Woolen Fabrics:
Basically, the woolen yarns are weaved using the plain weave, or sometimes the twill. Woolens are desirable for sportswear, jackets, sweaters, skirts, blankets, and similar general use. These fabrics are generally napped to give smooth and warmth effect.
5 .16 Weaving Worsted Fabrics:
Worsted yarns are chiefly by means of the twill woven. They are appropriate for tailored and dressy purposes, for spring and summer coats and suits, and for tropical suits.
6. PROPERTIES OF WOOL FABRICS
6.1 PHYSICAL PROPERTIES
| Strength | Among the other natural fibers, Wool is considered weak due to its amorphous structure. However it is possible to produce durable fabrics by carefully choosing the method of spinning and the weave. Strength is not an important factors for woolen fabrics intended for providing warmth. |
| Elasticity | Wool is an elastic fibre supported by its helix structure. It has natural three dimensional crimp which is helpful for its elastic recovery. Therefore, wool fibre has high elongation and also has very high elastic recovery. |
| Resiliency | Wool has high resiliency as compared to all fibers and thus exhibits good crease resistance. The fabric wrinkles less than other fabrics and also recovers fast if wrinkled. |
| Flexibility | As the crystallinity in fibre is less wool fibre has high flexibility which is an important factor in production of soft fabrics. |
| Abrasion resistance | Wool fabrics show low to medium abrasion resistance in case of apparel fabrics. High abrasion resistance is found in carpet wool. |
| Resilience | Wool fibre exhibits very high resilience. When crushed, it springs back to shape showing its exemplary resilience. Many structural aspects of wool fibre contribute to its resilience therefore, wool fabrics are more wrinkle free and even if wrinkled, ageing overnight makes it free from wrinkles. |
| Moisture Regain | Wool fibres can absorb large quantities of water as the fibres possess amorphous structure. Wool can absorb 13-16% of moisture under standard conditions. It may absorb more than 29% of its weight under saturation conditions. Due to its scaly nature wool fibre has natural water repellency. Water beads up on the surface of the wool fabric, thus making it suitable for light rainwear. One important aspect of wool is sorptive character is that it keeps the wearer warm even though it absorbs lot of water due to its very high heat of melting. |
| Dimensional Stability: | Wool fabrics show poor dimensional stability as the fibres interlock and shrink during processing, use and care of fabrics. Wool fabrics intended for making garments are finished for better dimensional stability. |
| 6.2 THERMAL PROPERTIES | Wool is not a good conductor of heat as it can hold air due to its crimpy structure. When made into woolen fabric, numerous air spaces withhold the heat, thus making it suitable for blankets. Wool fibre shrinks away when exposed to flame. It takes up flame and burns very slowly. It is self extinguishing when removed from flame and gives burnt hair smell. It leaves a black crushable bead as residue. As it does not support combustion, it used for fire resistant fabrics. |
| 6.3 CHEMICAL PROPERTIES | |
| Effect of alkalies | As wool is protein in nature, it is susceptible to damage by expose to alkalies. Wool fibre dissolves in 5% sodium hydroxide solution. |
| Effect of acids | Wool can withstand cold and dilute acids. Concentrated acids, mineral acids such as sulphuric and nitric acids can damage the fibre. |
| Effect of bleaches | Wool is damaged by sodium hypochlorite or other chlorine bleaches. Hydrogen peroxide or sodium perborate may safely be used. |
| 6.4 MISCELLANEOUS PROPERTIES | |
| Effect of Sunlight | Wool becomes weak with prolonged exposure to sunlight. |
| Effect of Mildew | Wool is generally resistant to mildew but if left continuously in damp condition, mildew may develop. |
| Effect of Moths | Wool fabrics are damaged by the larvae of moths as the sulphide linkage present in fibre is highly palatable. However, wool fabrics show resistance to silver fish. |
| Effect of Perspiration | Wool gets damaged if perspiration is left in the fabric as it is vulnerable for damage by alkalies. |
7. FINISHES GIVEN TO WOOL:
Wool fabrics are given the following finishes.
- Fulling – To make fabrics more fuller.
- Crabbing – To set the cloth and yarn twist permanently.
- Decating – To control shrinkage.
- London shrinking – superior pre shining treatment given to high grade wool fabrics.
- Moth proofing – To improve resistance to moths
- Napping – To make a fuzzy surface to improve comfort.
- Durable Press – To permanently set the fabric dimensions.
8. CARE OF WOOL FABRICS:
Wool fabrics can be maintained very well when drycleaned. Wet cleaning should be done carefully as wool is susceptible to shrinkage with uneven temperature, friction and moisture. During laundering, the fibres come closer and interlock with each other making it mat like. This property in wool is sometimes utilized for production of felt fabrics. Interlocking fibres together makes the fabric to shrink and loose shape. Careful handling of the fabric is solicited during laundering and storage.
The following should be avoided:
- Friction – (only kneading and squeezing to be employed)
- Use of alkaline soaps
- Use of alkaline reagents
- Use of chlorine bleaches
- Hanging on clothesline (dyeing on flat surface recommended)
- Dyeing in sunlight
- Storing damp clothes
9. CONSUMER PREFERENCE:
The majority of wool (72.8 percent) is used in apparel. Home furnishings account for 15.4 percent, industrial uses 6.7 percent, and exports 5 percent. Wool accounts for 3.3 percent of all fibers used for apparel.
The most important use of wool is for adult apparel – coats, jackets, suits, dresses, skirts, and slacks made from woven fabrics of varying weights; and suits, dresses, skirts, and sweaters made from knitted fabrics. All these gives the warm garments and with good tailored look.
In the home-furnishing area the major use of wool is in carpets and rugs where wool gives the more cover to the carpets and warm in the rugs.
Blends of different synthetic fibers with wool for suiting materials are increasingly important. They result in fabrics that are more appropriate in warmer conditions. Polyester is the most important fiber used in blending with wool.
Wool fabrics are preferred due to the following factors:
Wrinkle free fabrics
Sensorial comfort (touch)
Thermo-physiological comfort (providing warmth by holding water vapour to a large extent)
Permanent crease holding property when finished, suitable for suitings.
Excellent resiliency – less requirement for ironing.
Less soiling property.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- https://www.blackberry-ridge.com/prosdscr.htm
- https://www.woolmark.com/education/manufacturing/?enforce=true
- https://youtu.be/2FwX1Q1FnMU
ASSIGNMENTS
- Identify textile products made of wool fibers
- What are the finishes given to wool textiles?
- Collect pictures and swatches of wool and worsted
- Collect wool labels
SILK FIBER
1. INTRODUCTION:
Silk is an animal protein fibre obtained from silkworms. It is a very fine strand of solidified Protein secretion by caterpillars. Silk is a natural continuous lustrous and smooth filament fiber but irregular in diameter.
2. HISTORY OF SILK:
Silk is known as the ‘Queen Of Fibers’ as it is a strongest natural fibre, lustrous, smooth, with good affinity for dyes, highly absorbent, light weight, drapabitlity, elastic recovery. It is considered as a luxury fiber. Silk existed in China as early as 1725 B.C and the Chinese kept it as a secret for many centuries. As per the legend, Si-Ling-Chi, a Chinese princess was the first to find silk cocoons in her garden. She accidentally dropped a cocoon in warm water and while removing the cocoon, she found that it yielded a very fine fibre. Later Chinese learned to carefully nurture the silkworms on mulberry leaves and initiated ‘Sericulture’ and produced beautiful silk fabrics. From antiquity until the more recent establishment of the Chinese Republic, she was venerated as the Goddess of the Silkworm. Later Caravans introduced the silk in East and Alexander the Great carried this to Europe in 4th B.C.
After three thousand years of its origin the secret was stolen out of China. From then onwards silk became the pure prized fiber available naturally. Even though it has spread to many other countries, China still retains its first position maintaining monopoly in production of quality silk. India ranks second in production of silk rich fabrics.
3. SILK PRODUCING COUNTRIES:
Japan is the first country to produce the silk in large quantities, by using scientific means of production. Other silk producing countries are China, India, Italy, Spain, Bulgaria, Turkey, Greece, Syria and Brazil.
4. CULTIVATION OF SILK:
Sericulture:
The cultivation of silkworms for the purpose of producing silk filament is termed as Sericulture. ‘Bombyx mori’ is a species of moth that produces fine silk and suitable for sericulture. The life cycle of the silk moth has four stages.
- Egg – The female moth lays the eggs, which develops into the larva, or caterpillar – the silk worm.
- Silkworm – a caterpillar stage which eats on mulberry leaves.
- Cocoon- the silkworm spins a cocoon for protection at the end of its stage. The silkworm, development into the pupa, or chrysalis.
- Moth – the chrysalis inside the cocoons matures and emerges from the cocoon. The female moths lay eggs and the life cycle is continued.
Each stage of the silkworm is important and much care is taken by sericulturists by providing controlled atmosphere.
The life span of the moth is very short. Within 3 days after emerging from the eggs, the male and female moths mate and the female lays eggs. Infected eggs are removed. In a controlled atmosphere, each egg is hatched into a larva of 1/8th inch long. The larvae are carefully nurtured on chopped tiny mulberry leaves. As the larvae increase in size, they show voracious appetite and feed 3 times daily for about 20 to 30 days. The larvae changes its skin called as molting for four times before attaining the final size of around 3 ½”.
Its interest on food ceases and it shrinks in size and attains a pinkish hue and becomes transparent. The continuous realing of its head is an indication that it is ready for spinning its cocoon. At this time, those silkworms are transferred onto a bamboo structure, wherein they spin their cocoon within three days. Actually the larvae spin the cocoon by moving its head in figure ‘8’ motion from the outside of the body. As it spins the cocoons, larvae decreases in size and changes into the chrysalis. The silkworm extrudes the liquid fiber from two tiny orifices or spinnerettes in its head. As the liquid emerges into the air, it is hardened by a gummy substance called sericin (silk gum) which is extruded by two glands in close proximity to the cocoon.
At this stage, if the cocoons are left, the moth emerges out by dissolving the silk on the top of the cocoon. In order to get continuous filament, the moth should not emerge out. Therefore, the life cycle of the moth is terminated at this point by suffocating the chrysalis through heating the cocoons. After storing, the cocoons are safely marketedand then stored until they unreeled in preparation of yarn manufacturing.
http://www.csb.gov.in/gallery/album/mulberry-silkworm-life-cycle
http://silkwormmori.blogspot.in/p/movies.html
5. PROCESSING OF THE SILK FIBER:
5.1 Filature operations:
The cocoons that are raised by the silk farmers are delivered to a factory, called Filature, where the silk is unwound from the cocoon and the strands are collected into skeins.
The filature process includes the following steps.
(a) Sorting Cocoons:
The cocoons are sorted according to the color, size, shape and texture as all these affect the final quality of the silk. Cocoons generally range from white or yellow to grayish, depending on the source and the type of food consumed during the worm stage. Cocoons from China are white, Japanese cocoons are creamy white and yellow, Italian cocoons are yellow.
(b) Softening the Sericin:
Sorted cocoons are put through a series of hot and cold immersions, to soften the sericin. This helps in unwinding of the filament as one continuous thread. Raw silk consists of 80% fibroin (protein) and 20% of sericin (gum). At this stage only 1% of the gum is removed, because this silk gum is a needed protection during the further handling of the delicate filament.
(c) Reeling:
The process of unwinding the filament from the cocoon is called as reeling. As the filament of single cocoon is too fine for commercial use, three to ten strands are usually reeled at a time to produce the desired diameter of raw silk thread. The usable length filament may range from 300-600 metres.
(d) Throwing:
Silk filaments are combined and pulled onto the reel and twist can be inserted to hold the filaments together. This is called as throwing, which comes from an anglo-saxan word ‘thrawn’ meaning ‘to twist’. The resulting yarn is thrown yarn. Required number of strands are twisted together to form yarns of required size. If required, these yarns are doubled and twisted in opposite direction with another doubled yarn to produce different effects.
(e) Spinning:
Short ends of silk fibers from the outer and inner edges of the cocoons and from broken cocoons are spun into yarns in a manner similar to that used for cotton.
(f) Degumming:
Silk filament contains 80% of protein and 20% of sericin. The gum present on the surface of the filament has to be removed in order to bring out the natural luster of silk filament. Degumming is carried out by boiling silk yarn or fabric in a hot soap bath which will remove the sericin and other impurities but leave the silk unharmed. After removing the gum, silk looks creamy white in color, beautifully lustrous and luxuriously soft. Around 25 % of raw silk weight is lost during this operation.The presence of gum or sericin increases the tendency for the silk to water spot.
6. YARN COUNT OF SILK:
The size or the fineness of continuous silk filament or yarn is based on a direct system of weight known as ‘denier’. A denier represents the weight in grams of yarn of 9000 yards. The lesser the denier the finer the silk.
7. WEIGHTING:
Silk is priced based on its weight. As degumming reduces its weight, it is gained through a finishing process called weighting. Metallic salts such as stannous chloride are used for weighting colored silk which is carried out during dyeing process. It is followed by treatment with sodium phosphate. This is an accepted practice in silk industry as weighting imparts firmness and body to silk fabrics. Black silks are heavily weighted upto 15% than other colored silk that are weighed upto 10%. This is one of the reasons for less durability of black silks over the colored silks.
8. VARIETIES OF SILK:
| Cultivated Silk: | Silk obtained from cocoons of silkworms reared carefully through sericulture is called as cultivated or mulberry silk. |
| Wild Silk: | Silk produced by moths of species other than Bombyx mori. It is brown in color, more uneven and coarser. It is usually called Tussar Silk. The worms Antheria myletta are fed on oak trees, it assumes the natural brown color. Tussah silk is an important segment of the silk industry for production of low priced silks. However, these silks are not as lustrous as cultivated silks. |
| Waste silk or silk noil: | Short ends of textural spun yarns or in blends with cotton or wool. Sometimes called waste silk. |
| Raw silk: | Silk that has not had any degumming. This is popularly called as Silk-in-the-gum. |
| Spun Silk: | Yarns made from short fibers from pierced cocoons and short ends and outside and inside edges of the cocoons. |
| Reeled silk: | Continuous strands of silk obtained by combining the filament from cocoon, ready for twisting. |
| Dupion Silk: | Silk obtained from two silk worms that spin their cocoons together. The yarn is irregular in diameter and not continuous, but it may have good luster. At present in Indian market, the terms ‘Dupion silk’ is also applicable to spun silk obtained by spinning together staple silk obtained from pierced cocoons, doubled cocoons, floss brushed from cocoons before reeling, fleece from the beginning and end of each cocoon, scrap from the machine waste etc. |
| Pure dye Silk: | It indicates that weighting upto accepted levels alone has been used for silk. This dyed weighted silk is termed as pure dye silk. |
| Washable Silk: | Silk that can be easily washed and does not require dry cleaning. |
| Khadi Silk: | Silk that is hand spun and hand woven. The yarn/fabrics show irregularity and thick and thin places. |
| Ahimsa Silk : | Silk obtained from cocoons which are not stiffled to suffocate/ kill the chrysalis. |
9. VARIETIES OF SILK PRODUCED IN INDIA: Four varieties of silk are produced in India.
- Cultivated/ Mulberry Silk
- Tussar Silk/ Wild Silk
- Muga Silk (produced in limited quantities in North-East)
- Eri Silk – (produced mainly in North-Eastern states and also produced in AP)
10. PROPERTIES OF SILK FIBERS:
In spite of its high cost, silk has been one of the most popular fabrics because of its unique properties. Soft, supple, strong and lighter in weight than any other natural fiber, silk is prized for its lightness with warmth, sheerness with strength, and delicacy with resiliency.
10.1 PHYSICAL PROPERTIES
| Lustre | Amongst all natural fibres, silk is highly lustrous. Wild silk is not as lustrous as cultivated silk. |
| Structure |  Silk is a transparent filament with slightly irregular diameter. Silk from a cocoon has two filaments held together with sericin, the silk gum. Hence it appears wedge shaped in cross section. It is estimated to be 70-75% crystalline and 30-25 % amorphous. Silk is a transparent filament with slightly irregular diameter. Silk from a cocoon has two filaments held together with sericin, the silk gum. Hence it appears wedge shaped in cross section. It is estimated to be 70-75% crystalline and 30-25 % amorphous. |
| Strength | Silk is the strongest natural fiber. It has a high tenacity amongst the protein fibres because of high fibre orientation, hydrogen bonding and its continuous length. When compared to synthetic fibres, its tenacity may fall in mid-range. |
| Elasticity | Silk is an elastic fiber with medium elongation at break. At 2 % elongation the elastic recovery of silk is 90 %. It returns to its original size gradually and loses little of its elasticity. |
| Resiliency | The resiliency of silk is in medium range. But silk fabrics retain their shape and resist winkling. Heavily weighted fabrics wrinkle more and show decreased resilience. |
| Abrasion resistance | Silk has moderate abrasion resistance. Its smooth surface and wedge shape helps in resisting the abrasion. |
| Drapability | Silk has excellent drapability. The suppleness and pliability aided by its resilience and elasticity accounts for its drapability. |
| Density | Silk fibre has a specific gravity or density of 1.64 gm/cubic centimeter. It is possible to make light weight fabrics without sacrificing the strength. |
| Absorbency | Silk fabric can absorb water up to 1/3rd its weight without feeling wet to the touch. This property enables silk to take up dyes very well during dyeing and printing. |
| Dimensional stability | Silk fabrics retain their shape very well as the shrinkage in washing is found to be minimum. Crepe fabrics shrink more during laundering but can be restored back with careful steaming. |
| Cleanliness & Washability | Silk is a hygienic material because its smooth surface does not attract dirt. It can also be easily cleaned using mild soaps and in dry-cleaning. |
|
Shrinkage |
Silks show normal shrinkage because of its filament length & smooth surface. Hence they can be easily restored by ironing at moderate heat and damp conditions. |
| Heat Conductivity | Since silk is a protein fiber it is a nonconductor of heat like wool, hence it is used for winter apparel. |
10.2 THERMAL PROPERTIES:
Being a protein fibre, silk has a lower thermal conductivity than cellulosic fibres. However, it is possible to make comfortable fabrics by carefully choosing suitable fabric construction. Silk smolders while approaching flame; it burns slowly in flame giving a hair burnt smell and it does not support combustion. Therefore, it is self-extinguishing, leaving a crushable bead. Silk scorches if ironing temperature exceeds 150 Degree Celsius.
10.3 CHEMICAL PROPERTIES:
| Effect of alkalies | As a protein fibre, silk is vulnerable to alkalies. But the action of alkalies is slower than wool. It gets dissolved in caustic soda. Neutral soap is recommended for washing. |
| Effect of acids | Silk is resistant to organic acids and hence these are used in finishing silk fabrics. Like wool keratin, silk fibroin also gets damaged with mineral acids. Silk has the characteristic of absorbing and holding acid molecules which tend to damage silk fibroin on storage. |
| Effect of bleaches | Silk is resistant to mild bleaches such as hydrogen peroxide or sodium perborate. It gets damaged with the use of chlorite bleaches. |
| Affinity for dyes | Silk has very good affinity to acid dyes, but their light fastness is unsatisfactory. |
10.4 MISCELLANEOUS PROPERTIES:
| Effect of Sunlight | Direct sunlight damages silk hence shade drying is recommended. Raw silk is more resistant to light than degummed silk. |
| Effect of Mildew | Silk is not susceptible to mildew unless left in damp condition for a long time. |
| Effect of Moths | Silk has high resistance to moths and silver fish. But beetles may destroy the fibre. |
| Effect of Perspiration | Silk is damaged when perspiration is left in garments after washing. |
| Effect of Heat | Silk is somewhat sensitive to heat. It will begin to decompose at 3300 F. |
| Resistance to Mildew | Mildew does not affect silk unless left for some time in damp state or under the extreme conditions of tropical dampness. |
| Resistance to Insects | In wool and silk blends, the beetles and larvae of moths may affect it, which affects the wool. |
|
Resistance to Perspiration |
Silk fabrics are damaged by perspiration. The silk itself deteriorates and the color is affected, causing staining. |
11. FINISHES GIVEN TO SILK:
- Degumming – Removal of silk gum, sericin.
- Weighting- Adds weight after degumming.
- Scrooping – Treated with organic acids to produce the scroop sound when worn
- Calendering – Enhances luster and makes it wrinkle free.
12. CARE OF SILKS:
Silk fabrics are regarded as clean fabrics as they will not hold much soil due to their filament nature. Hence, cleaning of silks is simple and does not require any friction. Mild soaps such as reeta nut or neutral soaps / liquid soaps can easily be used for washing of silks.
Luxury silk fabrics have to be dry cleaned in order to retain their high luster and softness. Crepe, georgette and organzine fabrics need careful handling as they shrink in washing. The shape of these can be restored back by careful steaming.
13. USES OF SILK:
Silk is used primarily in apparel and home furnishing because of its appearance and cost. As silk is versatile, it used to create a variety of fabrics from sheer, gossamer chiffons to heavy, beautiful brocades and velvets. Silk is suitable for both warm weather and cold weather because of its absorbency and low heat conductivity. In furnishings, silk is often blended with other fibers to add a soft luster to the furnishing fabric. Silk blends are often used in window treatment and upholstery fabrics. Occasionally, beautiful and expensive handmade rugs will be made of silk.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- https://en.wikipedia.org/wiki/Silk
- https://texeresilk.com/article/silk_making_how_to_make_silksff.arts.ac.uk/Fibre%20Processing/silkprocessing.html
- http://sff.arts.ac.uk/Fibre%20Processing/silkprocessing.html
- www.madehow.com/Volume-2/Silk.html
- http://www.madehow.com/Volume-2/Silk.html
- https://youtu.be/-wbmEjTvszI
- https://youtu.be/vCZQ56oVfDM
ASSIGNMENTS
- Identify textile products made of silk fibers
- Collect information about local markets where silk products are sold
- Collect pictures of various silk products/ types from different parts of India
- Collect pure silk labels
MECHANICAL SPINNING
1. INTRODUCTION:
Spinning is a yarn making process which involves drawing and twisting a mass of fibres into a long, continuous thread. Yarn is the generic name given for an assemblage of fibres that is laid or twisted together.
2. TRADITIONAL SPINNING METHODS:
Spinning is also one of the inventions of primitive man besides wheel and fire. Very crude yarns were produced in those days which were the products of hand spinning. Even today this type of spinning is seen in India for making wicks. Spinning progressed with the invention of small tools like ‘Takli’ and ‘Charkha’.


Takli is a disc like device having a long rod with a small hook at the top. Charkha produces finer yarns than takli and even today charkha is the main tool of ‘Khadi’ industry.
Spinning was considered as only a hand method until the invention of spinning machinery in 18th century by an Englishman James Hargreaves. Later the machinery has taken so many shapes by several technicians and today the mills are highly equipped with highly technical automatic spinning frames which produce very fine yarns at higher speeds.
3. METHODS OF COMMERCIAL SPINNING:
Shorter fibres are spun into yarns by mechanical spinning while artificial fibres are spun by chemical spinning. All natural fibres except silk are staple fibres. Since silk is a filament fibre, simply twisting of few filaments together makes a yarn.
Short staple fibres such as cotton, wool, flax and other minor fibres undergo different operations while production of yarns. As length is a constraint in these fibres, sufficient pliability in the fibres is required. The convolutions of cotton, roughness of flax and scales of wool contribute to the cohesiveness of the fibre and thereby make it possible to produce yarns of different counts. Flexibility and uniformity in fibres improve the evenness and quality of yarns.
4. MECHANICAL SPINNING:
The process of developing short fibres into long continuous yarns involves several steps and fibres assume forms of lap, sliver, roving and finally yarn.
- Lap formation through blending and opening
- Lap to card sliver by carding process
- Card sliver to comb sliver by combing process
- Sliver to roving by drawing out/ drafting process
- Roving to a yarn by twisting process
- Reeling of yarn on bobbins, spools or cones
Cotton fibre lends itself to all the above processes, hence taken as example for the description of mechanical spinning process.
4.1 Opening, cleaning and blending
Different varieties of cotton after ginning arrive at the mills in the form of compressed bales. These bales are opened and blended for uniform quality in the final yarn. The fibre is fed into the opener and blender. These machines separate or open the hard lumps and blend the fibres from different bales. Cleaning of the fibres also takes place during this process as trash separates from the fibre.
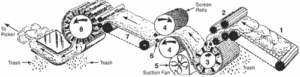
Several opening and blending machines with different technologies are prevalent in the industry. A typical machine consists of mechanical bale pickers that pick up layers of cotton from matted bales. The fibre is mixed and passed to an opener. Cylinders with protruding fingers open up the lumps and partially separate the remaining seeds, burs and trash from the fibre. The type and number of cylinders depend on the type and fineness of the fibre used. The commonly employed porcupine beater revolves at a speed of 1000 revolutions per minute. Cotton that emerges out of this process is slightly uniform and free from 1/3rd of the trash content. The sheet of cotton called ‘lap’ is conveyed over the belt ready for carding. Each lap is a loosely tangled mass of fibres of one inch thick and 40” wide.
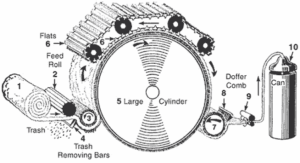 4.2 Carding
4.2 Carding
The purpose of carding is two fold
- Removal of further impurities/ trash in fibre mass.
- Arranging the tangled mass to assume parallel alignment, so that the longitudinal axes are somewhat parallel.
Carding machine consists of cylinders anchored with very fine hooks or wire brushes. A belt with anchored wire brushes moves slowly over the moving cylinder in concentric magnitude. The lap is passed through a beater section and drawn over the fast revolving cylinder. The two sets of pins/ brushes on cylinder and belt move in the same direction, but at different speeds to tease fibres into a filmy layer, so that a thin web of fibres is formed on the cylinder. The teasing action also removes remaining trash, disentangles the fibres and arranges them in a relatively parallel manner. The web thus formed is passed through a funnel shaped device that molds it in the form of soft round rope like mass called ‘card sliver’, about the diameter of ½ ” to ¾ “. Inexpensive carded yarns are produced from these slivers directly. For more serviceable yarns, further processing is done.
4.3 Doubling
The card sliver is not completely uniform in diameter and the fibres are not totally parallel. This necessitates doubling i.e., combining several card slivers together to make compact slivers.
4.4 Combing
When high quality cotton yarns with superior evenness, smoothness, fineness and strength are required, the fibres are combed. Combing process is an additional process that straightens the fibres, makes them parallel to each other and removes short fibres called noils.
Combing machine consists of several fine toothed combs with increasing speed. These combs continue straightening the fibres and arrange them with high degree of parallelism and separate the noils below ½ ” length. This process finally produces combed slivers made of long fibres, which, in turn produces a smoother and more even yarn. Above 25% of cotton is eliminated due to this process and this becomes a valuable byproduct used in non-woven industry. As combing influences the evenness, smoothness and strength of yarn, it is often indicated on fabric labels as ‘combed cotton’.
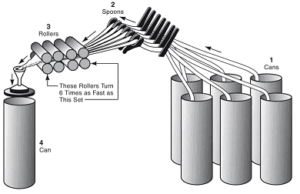 4.5 Drawing/ Drafting
4.5 Drawing/ Drafting
The slivers from different combing units or carding units are processed through the drafting frame. Eight slivers are drawn together to produce an attenuated ‘drawn sliver’ having the diameter of one card (comb sliver). In this process, fibres of different types can be blended together to form blended yarns. For example, to produce cotton polyester blend of 50:50, 4 cotton slivers and 4 polyester slivers are combined to form a single blended sliver.
The draw frame has several pairs of rollers; each advanced set revolves at a progressively higher speed. By pulling action of these cylinders, the slivers are attenuated into thinner and longer slivers, where in fibres assume parallel alignment. These drafted slivers are taken to a slubber where similar rollers attenuate the sliver further. The slub thus formed is passed to the spindles where first twist is imparted to the sliver to withstand the strain on roving frame and wound on bobbins.
4.6 Roving
Roving frame facilitates further drawing and twisting of the slivers from bobbins till the sliver attenuates to the size of a pencil lead. Two stages of roving are apparent: intermediate and fine. Even though operations are same, each operation produces a finer product than the received stock. Roving is the final product of the several drawing out operations and preparatory stage for final insertion of twist. Roving has no strength, as it is given only enough twist to keep the fibres together. It will break easily if pulled apart.
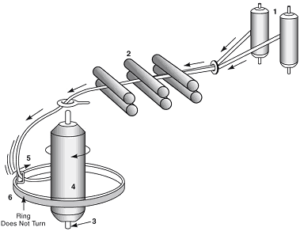 4.7 Spinning
4.7 Spinning
This is the final operation for formation of yarn of desired size. The spinning frames complete the manufacture of yarns by performing operations such as drawing the roving, inserting twist and winding yarn on bobbins, all in one operation.
The roving on bobbins is fed through sets of rollers on spinning frame. Just as in roving, the front set of rollers rotates faster than the back set. This difference attenuates the yarn and makes it even, smooth and uniform.
Two types of spinning frames are commercially employed. For relatively high speed production and making coarser yarns, ring frame is used. For finer yarns, mule frame is used, which operates at low speed. On ring frame, the attenuated yarn is fed down, guided through a ‘U’ shaped guide called a ‘traveller’ on to a take up package or bobbin. The traveller moves around the take up package at a slower speed than the take up package. Hence the name ‘ring spinning’. The movement of the traveller and the turning of the spindle on which the bobbin is held combine to introduce twist into yarns. The spindle revolves about 13,000 revolutions per minute. The size of the yarn and the amount of twist (turns per inch) can be controlled. The yarn on bobbins may be reeled into skeins or wound on spools for weaving and further processing.
Consumer should be aware that the strength and other properties of fabrics mainly depend on the yarn construction and fabric construction rather than the qualities of the fibre itself. The production of yarns depends on various factors such as its intended end use, required kind and quality of yarns, processing necessary to produce required size and amount of twist given to the yarns. For example, a towel material needs a thicker yarn with less twist; crepes need high twist; fine yarns are required for apparels. Thus the purpose of the yarns determines the kind and number of manufacturing operations required.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- Pizeto (1990) Fabric Science, AATCC, USA
- Wynne A (1997) Textiles The Motivate Series, Macmillan
- https://en.wikipedia.org/wiki/Spinning_(textiles)
- http://textilelearner.blogspot.com/2013/12/textile-spinning-process-of-cotton-yarn.html
- https://study.com/academy/lesson/what-is-spinning-in-textiles-definition-process.html
- https://www.textileschool.com/257/yarn-spinning/
- https://youtu.be/kH_b3Heo48I
ASSIGNMENTS
- List textile products made from fibers to yarn stage
- Collect information about mechanical spinning units at local areas
CHEMICAL SPINNING
1. INTRODUCTION:
Man-made and synthetic fibres are filamentous in form as their manufacturing process can be controlled at any stage unlike natural fibres. All synthetic fibres are produced from polymers. These are in solid form at room temperature often available in the form of chips/particles of one to two millimeters. The solid state of the polymer needs to be altered in order to reshape them to get the desirable shape. This is possible only when the solids are transformed into liquid form. The ‘free flowing’ polymer liquids are extruded through fine holes to form thin and long filament fibres.
2. METHODS OF CHEMICAL SPINNING:
Different methods are employed for converting the solid polymers into liquid form. Accordingly the extrusion also differs. Based on these, three methods can be evolved for production of man-made and synthetic fibres. These are termed as ‘chemical spinning’ or ‘extrusion’.
 2.1 Melt spinning:
2.1 Melt spinning:
Melt spinning is applicable to the polymers which are capable of melting at elevated temperatures. This is the simplest and most inexpensive process, as it involves only melting, extrusion and cooling. The polymer is melted, extruded through the holes of a spinnerette which is a perforated metal plate of small diameter. Each hole may be as small as 0.5 mm diameter or even smaller. Each spinnerette may contain 1 to 100 holes depending on the type of yarn or tow produced. A tow is a rope of filaments collected from different spinnerettes that are used for making staple fibres for blending.
 In the manufacture of melt spun filaments, the polymers in chip form are fed through a hopper and fall on a hot grid where it melts. The molten polymer is collected and filtered to remove suspended impurities that block the polymer flow and also vacuum treated to remove the air bubbles, so as to ensure continuous flow of polymer liquid that produces continuous filament.
In the manufacture of melt spun filaments, the polymers in chip form are fed through a hopper and fall on a hot grid where it melts. The molten polymer is collected and filtered to remove suspended impurities that block the polymer flow and also vacuum treated to remove the air bubbles, so as to ensure continuous flow of polymer liquid that produces continuous filament.
The polymer stream collected in a tank is forced through the holes of the spinnerette into a cooled air chamber which aids in solidification of the filament. The fibres that are produced by melt extrusion are polyamide, polyester and polypropylene.
The temperature employed for melt extrusion is generally 30°C above the melting point of the polymer, so that the viscosity of the fibre is low enough for extrusion. The viscosity of a molten polymer is also a measure of the length of the molecular chains. The higher the viscosity, the longer the polymer chain.
The filaments thus produced have the polymer chains arranged at random. To improve its orientation, the filaments are cold drawn. To facilitate cold drawing, filaments are drawn between two sets of rollers which operate at progressively higher speeds. The second set revolves at least 4 times the speed of the first set, so that drawing of the filament takes place. The randomly arranged molecular chains are oriented towards fibre axis and the filament becomes finer and stronger. This also facilitates the crystal formation when the polymer chains are drawn closer.
2.2 Dry/Solvent spinning:
When a polymer cannot transform into liquid through melting, an alternative method is employed, wherein the polymer is dissolved in a suitable solvent. If the solvent is volatile, it leaves the polymer in a desired shape when the solvent dries. Therefore this method is termed as dry spinning.
The polymer is dissolved in a solvent up to 25% and transformed into polymer solution. Sometimes the solution is heated to ensure sufficient flow of polymer through the tiny holes of the spinnerete. At this stage, certain additives like coloured pigments (spun dyed/dope dyed) can be introduced. Delustering agents such as titanium dioxide can also be added to the solution to produce dulled filaments. The polymer solution is filtered, vacuum treated to remove suspended particles that block the spinnerette holes and air bubbles that interrupt the formation of continuous filament.
The solution from the storage tank is forced through the tiny holes of the spinnerette into a heated air chamber where hot air or nitrogen is in circulation. The hot air evaporates the solvent from the polymer and carries it to another chamber wherein the solvent recovery takes place. After spinning, the filaments require washing.
This process is considered to be a costly one as it needs huge investment for a solvent recovery plant. About 10 % of the solvent is lost during this process, part of which may be present in these filaments. The solvents used may be expensive, toxic and flammable. Generally 3 to 6 kgs of solvent is used per kg of polymer. However, this process is considered to be fast (500-1000 metres per minute).
Unlike melt spinning, the cross sectional shape of the filament does not resemble the hole of the spinnerette as the drying up of solvent leaves the polymer in a different shape like a lobular shape in case of acetate fibre. Fibres such as cellulose diacetate, cellulose triacetate, polyacrylonitrile and modacrylics can be modified to become cellulose acetate and dissolved in a cheaper solvent such as acetone and on evaporation, acetate fibres are produced.
The cellulose acetate filaments generally are not drawn after spinning as the orientation of the molecular chains seems to be sufficient where as acrylic fibres are cold drawn like polyamide and polyester fibres to improve orientation. The cross sectional shapes of the filaments produced through melt spinning resembles the shapes of the holes of the spinnerette. Thus, the shapes of the fibres can be altered by changing the shapes of the holes to alter the lustre and bulking properties.
2.3 Wet spinning:
Wet spinning is applicable to those polymers which cannot be either melt or dissolved in a volatile solvent to change it from a solid to liquid state. A non-volatile solvent is used to change it to a liquid state. As the solvent can’t be evaporated, it is made to pass through a chemical bath, wherein the filament coagulates leaving the solvent, hence the name wet spinning.
Wet spinning is a slow process (30-80 metres/ minute) as the filament is regenerated through coagulation. As the coagulation takes place in a chemical bath, the spinnerettes should be made of corrosion resistant precious metal alloys, which again needs huge investment. The holes used are much smaller when compared to other two methods. Around 20,000 holes are present if the filament is turned into a tow.
The polymer solution is filtered to remove suspended particles and vacuum treated to free from air bubbles and then collected in tanks. Additives such as pigments and delustering agents can be added. Then it is forced through spinnerette into a chemical bath containing a dilute acid and gets coagulated in filament form. The filaments are then washed and drawn into desired diameter. Certain additives are introduced into the coagulating bath to alter the handle characteristics of the filaments.
Fibres that are manufactured using this method include viscose rayon and polyacrylonitrile. The non-volatile solvents are generally cheaper and therefore, recovery is not attempted. The cross sectional shapes of the filaments are often serrated (viscose rayon) due to chemical reaction that leads to coagulation of the filament.
3. DELUSTERING:
Fibres differ in lustre which depends mainly on the contour of the fibre. Smooth surface totally reflects the light that falls on it and makes it shiny. Highly lustrous fabrics are not always required, particularly if it is to be dyed. Therefore, it is necessary to make it dull. Titanium dioxide, a delustering agent is added to the polymer/dope solution before extrusion. The chemical is uniformly dispersed in the polymer. Under microscope Titanium dioxide is seen in the form of fine black spots. The presence of these spots makes the fibre dull by absorbing part of the light ray and reflects the rest.
4. DOPE DYEING OR SOLUTION PIGMENTING:
When single colored filament is required in large quantities, the pigments are added to the spinning solution before extrusion. This method of dyeing is much cheaper than the other methods as there is no wastage of color and also ensures good color fastness. However, utilization of one colored filament may be difficult.
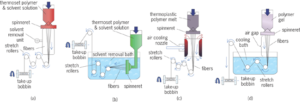 REFERENCES
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- Pizeto (1990) Fabric Science, AATCC, USA
- Wynne A (1997) Textiles The Motivate Series, Macmillan
- https://nptel.ac.in/courses/116102010/18
- https://en.wikipedia.org/wiki/Spinning_(polymers)
- http://www.tikp.co.uk/knowledge/technology/fibre-and-filament-production/wet-spinning/
- www.tikp.co.uk/knowledge/technology/fibre-and-filament-production/wet-spinning/
- https://textilestudycenter.com/melt-spinning-dry-spinning-and-wet-spinning-method/
- https://youtu.be/x0VFeMTSIb4
- https://youtu.be/cn6K1m7yH0I
ASSIGNMENTS
- List textile products made from fibers to yarn stage
- Collect information about chemical spinning units at local areas
- What are the fibers undergo chemical spinning? Enlist them
RAYON FIBER
1. INTRODUCTION:
Rayon fibre is a manufactured fibre composed of regenerated cellulose in purified form. It is the first manmade fibre that was commercialized as early as 1895. The probability of making an artificial fibre just like silk had been predicted by Robert Hooke in 1664. The credit of inventing rayon fibre in 1884 goes to Count Hilaire de Chardonnet who is regarded as “Father of Rayon’.
The first rayon was produced from nitrocellulose. Commercially viscose rayon became successful by 1919 and many factories mushroomed around the globe.
2. DEFINITION:
The Textile Fibre Product Identification Act (TFPIA) defines rayon as ‘a manufactured fibre composed of regenerated cellulose in which substituent’s have replaced not more than 15 per cent of the hydrogen’s of the hydroxyl radicals’.
3. PRODUCTION OF RAYON
The production of rayon basically is converting the cellulosic raw materials chemically into another form, which is then changed into cellulose again in purified form. Two principle types of rayon produced are:
- Cuprammonium
- Viscose rayon
High wet modulus rayon is also produced whenever more wet strength is required.
3.1 Viscose Process
The raw material used for this process is cotton lints or wood pulp made from specific wood types like spruce, hemlock, and pine trees.
- The wood is made into chips, cooked, bleached, treated and pressed and purified sheets of white wood pulp containing cellulose
- Steeping: The cellulose sheets are soaked in caustic soda (NaOH) to form alkali cellulose
- Pressing: The swollen alkali cellulose mass is pressed between rollers to remove excess fluid
- Shredding: These alkali cellulose sheets are broken into fluffy white flakes called cellulose crumbs
- Ageing: The cellulose crumbs are aged for 2 to 3 days under controlled humidity & temperature
- Xanthation: Carbon disulphide solution which is in light orange shade is added to the cellulose crumbs, which are transformed into cellulose xanthate, still in crumb form.
- Dissolving: The xanthate crumbs are dissolved in a weak solution of caustic soda and transformed into a thick viscose solution resembling honey colour & consistency.
- Ripening: The viscose solution is set to stand for a period of time, allowing it to ripen
- Filtering: The viscose is filtered to remove any undissolved particles that might haper spinnerette extrusion
- Degassing: Aged and filtered viscose is treated to remove water bubbles which interfere with its formation into continuous filament. This process is called degassing.
- Spinning: The dope is forced through the holes of a spinneret into an acid bath wherein it gets coagulated in a linear filamentous form.
- Drawing: The rayon filaments are stretched, known as drawing, to straighten out the fibers. It improves the orientation there by the strength & modulas of rayon filaments.
- Washing: The fibers are then washed to remove any residual chemicals
- Cutting: The filaments are cut down when producing staple fibers
- The filaments are doubled and drawn to spin them into fine yarns. The filaments that leave the coagulation bath are put through three methods of processing.
- Pot or box spinning: The filaments are led through a funnel moving up & down in a cylinder called topham box which revolves making the filament to deposit along the walls of the cylinder forming a hollow cake. It is removed, washed, treated with chemicals to remove impurities, rinsed, dried and wound on spools.
- Spool spinning: The filaments are made to pass over the rollers and wound on perforated cylinder which later facilitates its washing and other processing and finally wound on spools.
- Continuous Process: The filaments are put through a continuous process of washing and subsequent processing and wound on desired package.
4. PROPERTIES OF RAYON: The properties of viscose rayon and high-wet-modulus rayon differ in their structure, properties and intended end use.
4.1 PHYSICAL PROPERTIES:
| Structure | Viscose rayon is characterized by discontinuous longitudinal lines called striations which are a result of its ‘serrated’ cross sectional shape due to the loss of zinc sulphate during coagulation. As zinc sulphate is less in the coagulating bath of high wet modulus, the shape is round and thereby no striations are present. The degree of polymerization is 300 to 400 as against 10,000 for cotton. Though both are cellulosic fibres, differences occur in their properties because of the difference in degree of polymerization. |
| Luster | Rayons by nature are very bright and their brightness is controlled by the addition of titanium dioxide making it semi-dull and dull depending on the quantity added. |
| Density | The density of rayon is 1.50 gm/cubic cm as the basic material is cellulose in its purified form. |
| Strength | Viscose rayon is a medium strong fibre having slightly better strength than wool but inferior to cotton and silk. Rayon looses its tenacity when wet, up to 50 % of its dry tenacity due to its amorphous nature which allows it to absorb more water, thereby facilitating the slippage of molecular chains. |
| Elasticity | Viscose rayon has great extensibility of around 15 to 20 % when dry and wet but poor in their elastic recovery. |
| Resiliency | The resiliency of viscose rayon is low when compared to wool and silk. The fabrics are soft but readily crease during use. |
| Moisture Regain | Viscose rayon is one of the most absorbent fibres, better than cotton and linen due to its amorphous structure, exceeded by only wool and silk fibres. This property coupled with good heat conductivity enables rayon fabrics to be comfortable during summer. Care should be taken not to sag these fabrics when wet due to loss in strength. |
| Dimensional Stability | Like cotton, rayon fabrics show more of relaxation shrinkage and shrink more than cottons. So finishes are imparted to control relaxation shrinkage. |
| Drapability | The probability of making tightly woven fabrics coupled with weight makes the rayon fabric highly suitable for draperies. |
4.2. THERMAL PROPERTIES:
Viscose rayon is a very good conductor of heat and thus very ideal for summer wear. As cellulosic fibre, it takes up the flame readily and burns faster than cotton and leaves a grayish white fluffy ash residue. As with cotton, it also shows an afterglow. Viscose rayon starts decomposing over 150 0C; ironing should be done at low temperatures.
4.3 CHEMICAL PROPERTIES:
| Effect of alkalies: | Even though rayon is cellulosic, its resistance to alkalies is inferior when compared to cotton. Concentrated alkalies disintegrate viscose rayon; therefore, mild soap is recommended for washing. |
| Effect of acids: | Like cotton, rayon also disintegrates in acids. It cannot withstand hot dilute and cold concentrated acids especially mineral acids. |
| Effect of bleaches: | During normal use, rayon does not discolor and retain its white color. If bleaching is required, household bleaches such as sodium hypochlorite (javelle water) sodium perborate and hydrogen peroxide at lower concentration can safely be employed. |
4.4 MISCELLANEOUS PROPERTIES:
| Effect of Sunlight | Compared to all natural fibres, rayon possesses good resistance to sunlight. However, prolonged exposure to sunlight may turn white fabrics into yellow and causes subsequent deterioration. |
| Effect of Mildew | Being pure cellulosic fabric, it becomes a good host for the growth of moulds. The fabric should not be kept under damp condition for long period. |
| Effect of Moths | Viscose rayon is not affected by cloth moths. However, silver fish may damage the fabric. |
| Effect of Perspiration | Rayon is fairly resistant to deterioration due to perspiration. More than the fabric, the color gets affected. |
5. FINISHES GIVEN TO RAYON:
- Calendering- for smooth finishing.
- Preshrinking- for providing good dimensional stability.
- Flame retardancy- to control the rate of burning and make it fire protected.
- Embossing- for providing decorative effects.
- Stiffening – for providing body.
- Wrinkle resistance – for good shape retention.
- Water repellency – rarely given to make it suitable for rain wear.
6. CONSUMER PREFERENCE:
- Viscose rayon fabrics are lustrous as compared to cotton and other cellulosics and resemble silk.
- Economical when compared to silk and thus sometimes referred to as ‘poor man’s silk’.
- Versatile as the fabric is suitable for many types of apparels.
- Comfortable as it possess good absorbency as well as good heat conductivity.
- Highly suitable for durable draperies as viscose rayon shows good drapability coupled with good sunlight resistance.
- Easy to wash as it’s smooth surface does not attract dust and iron readily.
- Possible to make durable fabrics by carefully choosing the ply yarn and fabric construction.
- Excellent effect of colors and prints on rayon makes them suitable for various end uses.
- Viscose rayon provides softness when blended with other fabrics
- Slippage at seams may be a problem when tailored into garments which needs careful handling.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi.Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- https://en.wikipedia.org/wiki/Rayon
- https://www.britannica.com/technology/rayon-textile-fibre
- http://www.madehow.com/Volume-1/Rayon.html
- www.fibersource.com/fiber-products/rayon-fiber/
- http://www.fibersource.com/fiber-products/rayon-fiber/
- https://youtu.be/f_AvqnMTJjg
ASSIGNMENTS
- Identify textile products made of rayon fibers
- What are the apparel brands producing/ marketing Rayon products in India
- What are the finishes given to rayon textiles?
- Discuss about comfort provided by cotton and rayon products
- Identify and differentiae silk and rayon products at local market
HIGH WET MODULUS RAYON (HWM) FIBER AND CUPRAMMONIUM RAYON FIBER
1. INTRODUCTION:
HIGH WET MODULUS RAYON (HWM) FIBER
High wet modulus rayon (HWM) is a modified version of viscose that has a greater strength when wet. It also has the ability to be mercerized like cotton. HWM rayons are also known as “polynosic.” Polynosic fibers are dimensionally stable, and do not shrink or get pulled out of shape when wet like many rayons. They are also wear resistant and strong while maintaining a soft, silky feel. They are sometimes identified by the trade name Modal.
2. MANUFACTURING PROCESS: The process for manufacturing high-wet-modulus rayon is similar to that used for making regular rayon, with a few exceptions.
First variations: When the purified cellulose sheets are bathed in a caustic soda solution, a weaker caustic soda is used when making HWM rayon.
Second Variation: Neither the alkali crumbs nor the viscose solution is aged in the HWM process.
Third variation: when making HWM rayon, the filaments are stretched to a greater degree than when making regular rayon.
Fig. After the syrupy viscose solution is prepared, it is forced through a spinneret into an acid bath. The resulting strings or filaments are then stretched on godet wheels to strengthen them and put into a spinning Topham box. This method produces cake-like strings of rayon, which are washed, rinsed, and dried before being wound on spools or cones.
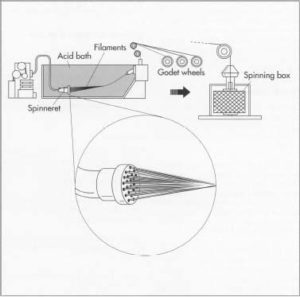
3. QUALITY CONTROL
As with most chemically oriented processes, quality control is crucial to the successful manufacture of rayon. Chemical make-up, timing and temperature are essential factors that must be monitored and controlled in order to produce the desired result.
4. END USES:
It is widely used in clothing as a replacement for cotton. Modal may be used on its own or in a blend with cotton, wool and other synthetic fibers viz. spandex etc.
- It is very soft and thus is popular for both clothing and house hold textiles.
- Used for tablecloths and bed linen (beddings), bathrobes, upholstery and in home furnishings. Also used as outerwear, sportswear and leisurewear.
- Also find applications in undergarments and toweling purposes.
- HWM is used exclusively for soft flowing tops and lingerie; exclusively in knitwear markets having high‐end apparel/non apparel products.
- For socks and stockings, as well as in technical applications, such as tire cord, abrasive ground
fabric, rubber cloths and other coating supports
5. BLENDS: HWM is blended with many fibers giving them the following basic properties.
5.1 HWM and cotton – With medium grade cottons it is the ability to import a combed cotton look and attractive
5.2 HWM and polyester – Gives standard and the high extensibility to the blend
5.3 HWM and wool – Shrinkage reduction with the use of minor proportions of the very high modulus fibres
6. CUPRAMMONIUM RAYON
Like Viscose Rayon, cuprammonium rayon is also a regenerated cellulose fibre. Cotton linters are used as the source of cellulose for this rayon. Cuprammonium rayon is made from reaction of Cellulose with copper salt and ammonia. After bleaching cellulose is added in ammonia solution of copper sulphate resulting in formation of cuprammonium cellulose which is spun into water and the yarn is washed with acid to remove traces of ammonia and dried.
Ammonia copper oxide solution is also known as cuprammonium hydroxide solution. Cuprammonium hydroxide solution is a solvent for cellulose. When a solution of cellulose in cuprammonium hydroxide is diluted with water or treated with dilute sulphuric acid, the cellulose is regenerated or re-precipitated. By using a spinneret, filaments of this regenerated cellulose can be produced.
7. MANUFACTURE OF CUPRAMMONIUM RAYON
The source of cellulose for this rayon is cotton linters, the purification of cotton linters is carried out in two stages:
- Mechanical Treatment
- Chemical Treatment
7.1 Mechanical Treatment: The cotton linters are transported in bales in highly compressed state and the object of the mechanical treatment is to loosen them and to remove mechanically admixed and loosely bound impurities such as dust sand, seed residues etc.
7.2 Chemical Treatment: The mechanically opened and purified cotton linters are boiled under pressure for several hours with dilute soda ash ( Na2Co3) solution (2%) to which a little amount of caustic soda may be added. The natural fatty matter present in the cotton is converted into soluble substance by the action of soda ash and thus removed from cotton linters.
7.2.1 Dissolution of Cellulose: In this, a solution of hydrated copper sulphate in 300-400 liters of water is introduced in a vessel at ordinary temperature with stirring. Some sugar is also added followed by caustic soda solution to form copper hydroxide.
Ground linters suspended in water are added to the above mixture to form copper cellulose.
The copper cellulose is filtered to remove the liquid, well ground and dissolved in a solution of ammonia in water.
7.2.2 Spinning Solution: By adding certain compounds to the cuprammonium cellulose solution, the solution is made more suitable for spinning. These compounds include glycerine, glucose, tartaric acid, citric acid, oxalic acid, cane sugar etc.
7.2.3 Stretch Spinning: In the spinning process, the cuprammonium cellulose solution is discharged through spinnerette into a solution of sulphuric acid in the form of relatively thick threads which are subsequently pulled (stretched) to very fine filaments.
8. PROPERTIES OF CUPRAMMONIUM RAYON
- The one important characteristic of these fibres is their extreme fineness. Filaments as fine as 1.33 deniers are produced regularly (as compared to viscose rayon which have a usual denier of around 2.5). This increased fineness is due to the stretch that is applied to the filaments during spinning.
- Because of its fineness, cuprammonium rayons produce a soft silk like handle.
- It has all the properties of cotton except that the average DP is lower and a larger portion of this fibre is occupied by amorphous regions. Hence the rayon swells to a greater extent and hence chemical reactions take place faster in the case of rayon than in case of cotton.
- Like viscose rayon it burns rapidly and chars at 180 deg C. It is degraded and weakened by exposure to sunlight in the presence of oxygen and moisture. On ignition, it leaves behind ash containing copper.
- The average tensile strength of cuprammonium rayon is 1.7-2.3 in dry and 0.9-2.5 in wet state.
- It has an elongation at break of 10-17% when dry.
- Moisture content at 70 deg F and 65% RH is about 11% as in case of Viscose Rayon.
- Dye absorption power for direct dyes of cuprammonium rayon is greater and shades obtained are deeper than viscose rayon.
- The filaments appear uniform with surfaces having no markings, in the longitudinal view. Cross sections are round and smooth with occasionally slightly oval.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi.Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- http://iupac.org/publications/pac/pdf/1967/pdf/1403×0337.pdf
- http://www.defaultlogic.com/learn?s=Modal_(textile)
- http://textilelearner.blogspot.com/2012/03/rayon-fiber-characteristics-of-rayon.html
ASSIGNMENTS
- Identify textile products made of different rayon fibers
- Discuss the difference between types of rayon textile products
POLYESTER FIBER
1. HISTORY
Polyester is one among the first developed petroleum based synthetic fibres next to nylon. It was first identified by the Dupont team of scientists under the leadership of Dr.W.H.Carothers along with polyamide fibre nylon. Scientists from Great Britain focused their interest in developing long chain linear polyester polymers. J.R.Whinfield and J.T.Dickson from calico Printers Association are responsible in bringing up the successful polyester fibre in 1941 called ‘Terylene’. It was made available to American Consumer from 1951 onwards under the trade mark ‘Dacron’. These fabrics found immediate consumer acceptance because of their ease of maintenance and excellent wrinkle resistance recovery.
2. DEFINITION:
The Textile Fibre Product Identification Act (TFPIA) defines polyester as ‘a manufactured in which the fibre forming substance is any long-chain synthetic polymer composed of atleast 85 per cent by weight of an ester of a substituted aromatic, carboxylic acid, including but not restricted to substituted terephthalic units’.
3. MANUFACTURING OF POLYESTER FIBRES
The manufacture of polyester is a simple process of allowing reactions between a dihydric alcohol and dicarboxylic acid for formation of a polymer and melting and forcing it through the holes of a spinnerette.
3.1 materials: Ethylene glycol and dimethyl terephthalate or terephthalic acid, petroleum by products.
3.1.1 Process:
- The two chemicals ethylene glycol and terephthalic acid are combined in an autoclave which is like a huge pressure cooker. At high temperatures, both the chemicals react with each other and a polymer that contains about 80 to 100 repeating units is formed.
- The polymer is extruded in a ribbon form which is removed and made into chips or pellets. Sometimes it can be stored in this form and shipped to the spinning mills.
- The chips/pellets from different autoclaves are placed in a hopper and mixed to ensure uniformity.
- The chips are made to fall on a hot grid which makes the polymer to melt.
- The molten polymer is filtered to remove impurities and passed through a vacuum to suck the air bubbles which interfere in formation of continuous filament.
- The purified polymer is forced through the holes of a spinnerette into cool air and then get solidified (melt spinning)
- It is stretched or cold drawn to orient the molecules in the fibres in order to improve strength and fineness. The amount of drawing depends on the end uses of the fibre. Majority of the yarn produced will be utilized for production of textured yarns.
- Generally polyester polymer is drawn 5 times their original length.
3.2 SPINNING OF POLYESTER FIBRES:
The drawn filaments are given slight twist and wound on spools. Each yarn contains many filaments, the number of which depends on the number of holes present in a spinnerette. Based on the end use, the filament yarns are given varied twist and made into versatile yarns.
Due to the desirable characteristics found in polyester, it is very common to find polyester as one of the principal fibres. Thus it is regarded as a ‘big mixture’ in the textile industry. To facilitate blending, the filaments from various spinnerettes are collected and made into a ‘filament tow’ which is textured (crimpy, wavy or coiled) between rollers at high temperature and cut into staple lengths (1″ to 5″).
4. PROPERTIES OF POLYESTER:
| 4.1 PHYSICAL PROPERTIES Structure |
Polyester fibres are rod like cylindrical in shape with uniform diameter and smooth surface. The pitted appearance found in many polyesters is due to the use of titanium dioxide to control its luster. The cross sectional shape is generally round and may show trilobal, T-Shape, pentalobal and trilateral in special polyesters.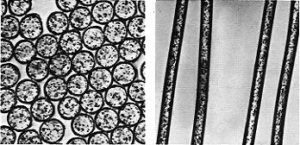  |
| Density | The density of polyester is 1.38. It can be made into a variety of fabrics ranging from light weight to heavy fabrics. |
Physical Properties:
| Strength | Polyester is a strong fibre when compared to the natural fibres. Its strength can be controlled based on the intended end-use of the fibre in the range of 2.5 gm to 9.5 gm/denier. Fibres with lower tenacities are utilized for apparels. Fibres having more than 7 gm/denier tenacity are utilized for industrial applications. While blending polyester with other fibres, strength will also form an important criterion. |
| Elasticity | Polyester is one of the textile fibres that exhibit good elongation and elastic recovery. The elongation is more in case of fibres of low tenacity and it is less when the strength is high. Polyester fibres exhibit high recovery under low stress. Recovery is 97 % at 2 % elongation. |
| Resiliency | Polyester has excellent resilience. This property helps in resisting wrinkles and provide excellent recovery if creased. |
| Abrasion resistance | Polyester has very good abrasion resistance. |
| Moisture Regain | Polyester is a hydrophobic fibre and has a regain of 0.4 % at standard conditions. Even at high humidity conditions, it has the moisture regain of 0.6 to 0.8 %. But the fibre has ‘wicking’ property which means moisture travels on the surface of the fibre, so as to keep the wearer comfortable. |
| Dimensional Stability | Polyester fabrics are given a finish called ‘heat setting’ which is a process of setting the dimensions of the fabrics, so that the fabrics neither stretch nor shrink. |
| Drapability | Polyester has satisfactory drape. The drape improves with the change in cross sectional shapes. Heavy polyesters drape well making it suitable for economical draperies. |
4.2 THERMAL PROPERTIES:
The heat resistance in polyester is high. The heat conductivity depends on the type of fabric construction. Polyester fibre shrinks away from flame as it is a thermoplastic fibre. It takes up flame readily and burns fast. It continues to burn after removal from flame with an aromatic odour leaving a hard bead residue. Polyester starts melting from 2380C to 2900C. Ironing temperature of 120 Degree Celsius is recommended.
4.3 CHEMICAL PROPERTIES:
| Effect of alkalies: | Polyester is resistant to mild alkalies but concentrated alkalies at high temperatures disintegrate the fibre. |
| Effect of acids: | Polyester’s resistance to organic acids and mineral acids is good to excellent. Highly concentrated mineral acids disintegrate polyester fibres if exposed to high temperatures. |
| Effect of bleaches: | Polyester is resistant to many household bleaches. It can safely be bleached without any deterioration. Polyesters are produced with optical brighteners which give permanent white color. |
Miscellaneous properties:
Effect of Sunlight: Polyester is highly resistant to ultraviolet rays and sunlight.
Effect of Mildew: Polyester’s resistance to mildew is very high.
Effect of Moths: Moths, silver fish and carpet beetles have no effect on polyester.
Effect of Perspiration: Perspiration has no effect on polyester.
Electrical Conductivity: Polyester is a poor conductor of electricity. Electricity produced on the surface due to rubbing with other objects will not be conducted away and tend to pile on the surface. It is termed as ‘static electricity’ which poses problems in low humid conditions.
Pilling: The formation of pills on the surface of the fibre due to enlargement of fibre ends is a problem in spun polyester fabrics.
5. FINISHES GIVEN TO POLYESTER:
- Heat Setting- for permanent shape retention and wrinkle resistance.
- Calendering- for smoothness and reduced pilling.
- Antistatic finish- for reduced electrical buildup.
- Embossing- for producing raised design for aesthetic look.
- Singeing– for producing smooth surface by burning off fibre ends.
- Soil repellency – helps in easy removal of all stains including oil.
6. CONSUMER PREFERENCE:
- Versatility of Polyester: Polyester is blended with almost all fibres and produce fabrics of desirable characteristics.
- Ease of acre: Due to its hydrophobicity it can be cleaned easily and dries faster.
- Wrinkle free fabrics: Keeps up good shape and does not require much ironing.
- Highly durable: It is resistant to most outside elements such as sunlight, acids in the air, bleaches and dry cleaning solvents..
- Very Economical: Polyester fabrics and its blends are available at moderate rates within the reach of all people.
7. USES OF POLYESTER:
- Polyester is used for making woven fabrics –possible to produce blends in 65:30 P:C or 50:50 P:C ratios. Blends are used for apparel, home furnishing
- Polyester is used for making knitted fabrics
- Polyester is used as fibre fill – Used in pillows, comforters, bedspreads, other quilted household and apparel fabrics, and winter jackets.
- Polyester is used to make Nonwovens – Sewn-in interfacings, fusible interfacings, pillow covers, and mattress inter-linings are examples of uses for nonwoven polyester fabrics.
- Polyester is chosen for many other consumer and industrial uses – pile fabrics, tents, ropes, cording, fishing line, cover stock for disposable diapers, garden hoses, sails, seat belts, filters, fabrics used in road building, seed and fertilizer bags; artificial arteries, veins, and hearts; and sewing threads.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi.Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- https://youtu.be/kb4tCcnA6jo – fiber identification test
- http://www.madehow.com/Volume-2/Polyester.html
- www.madehow.com/Volume-2/Polyester.html
- https://www.sciencedirect.com/topics/chemistry/polyester-fiber
- https://www.textileschool.com/234/polyester-fiber-and-its-uses/
- https://en.wikipedia.org/wiki/Polyester
- http://textilelearner.blogspot.com/2011/07/polyester-fiber-characteristics-of_11.html
ASSIGNMENTS
- Identify textile products made of different fiber fibers
- Discuss the different non apparel products made of polyester
- Why Polyester is called as ‘Big mixture’ or ‘Easy Care fabrics’
NYLON FIBER
1. INTRODUCTION:
Nylon is a man-made synthetic fiber that is strong while very light in weight, properties that lead to a wide variety of uses, such as fabric, rope and luggage. Nylon is a manufactured fiber in which the fiber forming substance is a long-chain synthetic polyamide in which less than 85% of the amide-linkages are attached directly (-CO-NH-) to two aliphatic groups.
Nylon is a synthetic polymer, a plastic, invented on February 28, 1935 by Wallace Carothers at the E.I. du Pont de Nemours and Company of Wilmington, Delaware, USA. The material was announced in 1938 and the first nylon products; a nylon bristle toothbrush made with nylon yarn (went on sale on February 24, 1938) and more famously, women’s stockings (went on sale on May 15, 1940). Nylon fibres are now used to make many synthetic fabrics, and solid nylon is used as an engineering material.
2. DEFINITION:
Federal Trade Commission Definition for Nylon Fiber is a manufactured fiber in which the fiber forming substance is a long-chain synthetic polyamide in which less than 85% of the amide-linkages are attached directly (-CO-NH-) to two aliphatic groups.
3. NYLON FIBER PRODUCTION:
Nylon fiber is produced by pushing molten nylon through tiny openings in a device called a spinneret; the nylon pieces then harden into a filament after they are exposed to air. These filaments are formed into bobbins and stretched once they have cooled down. A process known as drawing unravels the filaments or yarn and winds them into another spool; this procedure makes the molecules in the filament form parallel lines, which provides the nylon fiber with its elasticity and strength.
The term nylon refers to a family of polymers called linear polyamides. There are two common methods of making nylon for fiber applications. In one approach, molecules with an acid (COOH) group on each end are reacted with molecules containing amine (NH2) groups on each end. The resulting nylon is named on the basis of the number of carbon atoms separating the two acid groups and the two amines. Thus nylon 6, 6 which is widely used for fibers is made from adipic acid and hexamethylene diamine. The two compounds form a salt, known as nylon salt, an exact 1:1 ratio of acid to base. This salt is then dried and heated under vacuum to eliminate water and form the polymer.
In another approach, a compound containing an amine at one end and an acid at the other is polymerized to form a chain with repeating units this nylon is referred to as nylon 6.
4. CHARACTERISTICS OF NYLON FIBER
4.1 PHYSICAL PROPERTIES
Tenacity: 4-9 gm/den (dry), in wet 90% of dry.
Elasticity: Breaking extension is 20-40%.
Stiffness: 20-40 gm/den.
Moisture regain: 3.5-5%; (not absorbent due to crystallinity).
Specific gravity: 1.14.
Abrasion resistance: Excellent.
Dimensional stability: Good.
Resiliency: Excellent.
Softening point: Nylon 6,6 – 2290C, Nylon 6 – 1490C.
Melting point: Nylon 6,6 – 2520C, Nylon 6 – 2150C.
Hand feel: Soft and smooth
Lustrous: Like silk
Easy to wash: Easy Care, durable
Heat conductivity: Spun yarns lend fabrics light weight and warmth
4.2 CHEMICAL PROPERTIES
Acid: Nylon 6,6 is attacked by mineral acids is disintegrated or dissolved almost. It is dissolved in the concentrated formic acid. Nylon 6 is attacked by mineral acid but resistant to dilute boiling organic acid.
Bleaches: Not attacked by oxidizing and reducing bleaches but may be harmed by chlorine and strong oxidizing bleaches.
Alkali: Nylon is substantially inert to alkalis.
Organic solvent: Most of the solvent have little or no effect on nylon. Phenol metacressol and formic acid dissolve the fibre but solvents used in stain removal and dry cleaning do not damage it.
Light: No discoloration. Nylon 6 gradually loss of strength on prolonged extension.
Biological: Neither micro organism nor moth, larvae attack nylon.
Electrical: High insulating properties leads to static charges on the fibre.
Flammability: Burns slowly.
5. USES:
Apparel: Blouses, dresses, foundation garments, hosiery, lingerie, underwear raincoats, ski apparel, windbreakers, swimwear, and cycle wear .
Home Furnishings: Bedspreads, carpets, curtains, upholstery
Industrial and Other Uses: Tire cord, hoses, conveyer and seat belts, parachutes, racket strings, ropes and nets, sleeping bags, tarpaulins, tents, thread, mono filament fishing line, dental floss.
6. GENERAL NYLON FIBER CARE TIPS
- Most items made from nylon can be machine washed and tumble dried at low temperatures. Use warm water and add a fabric softener to the final rinse cycle.
- Remove articles from dryer as soon as tumbling cycle is completed.
- If ironing is required, use warm iron. (For specific care instructions, refer to garment’s sewn-in care label.)
7. ENVIRONMENTAL IMPACT, INCINERATION AND RECYCLING:
Berners-Lee calculates the average greenhouse gas footprint of nylon in manufacturing carpets at 5.43 kg CO2 equivalent per kg, when produced in Europe. This gives it almost the same carbon footprint as wool, but with greater durability and therefore a lower overall carbon footprint.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- Wynne A (1997) Textiles The Motivate Series, Macmillan
- https://youtu.be/3QSTE5wgZBU – Rayon & Nylon production video
- https://en.wikipedia.org/wiki/Nylon
- https://pubs.acs.org/doi/abs/10.1021/ie50372a007?journalCode=iechad
ASSIGNMENTS
- Identify textile products made of nylon fibers
- List different synthetic fibers and their basic products.
ACRYLIC FIBER
1. INTRODUCTION:
According to the definition set forth by the FTC an acrylic fiber is “any long-chain synthetic polymer composed of at least 85 % by weight of acrylonitrile units”. Acrylic fibers are soft, warm, lightweight, and resilient. It is mostly commonly used as a substitute to wool. As it is a thermoplastic fiber, it can be given crimp like wool and also cut to the same staple length as wool. They make easy-care fabrics.
2. HISTORY OF ACRYLICS:
Acrylonitrile, the substance from which acrylic fibers are made and from which the generic name is derived, was first made in Germany in 1893. It was one of the chemicals used by Carothers and his team in the fundamental research done on high polymers for the du Pont Company. Du Pont developed an acrylic fiber in 1944 and started commercial production of this fiber in 1950. The fiber was given the trade name Orlon. Three other companies began to produce acrylics: Chemstrand Corporation (now called Monsanto Fibers) introduced Acrilan in 1952, Dow Chemical (BASF Fibers) began the production of Zefran in 1958, and American Cyanamid began the production of Creslan in 1958. These same four companies were producing acrylic fibers in 1986.
3. METHOD OF MANUFACTURE:
3.1 Raw Material:
Acrylonitrile is made from acetylene or from ethylene which are petroleum derivatives. Chlorohydrin is formed by treating ethylene with hypochlorous acid. The chlorohydrin is reacted with sodium hydroxide to form ethylene oxide. Hydrochloric acid is added to ethylene oxide producing cyanoalcohol which is dehydrated to yield acrylonitrile.
3.2 Fiber Formation Process:
- Acrylonitrile is the raw material for manufacture of acrylic fibre by addition polymerization.
- The polymer is dissolved in solvents such as dimethyl formamide or dimethyl acetamide and made into spinning solution.
- The dope or the spinning solution is filtered to remove impurities and vacuum treated to make it free from air bubbles, so that extrusion of continuous filament is possible.
- By adding a delusterant, Orlon can be made semi dull.
- The dope is made to pass through the holes of spinenrette onto a hot chamber (Dry Spinning).
- The filaments are solidified by evaporation of the solvent.
- Solvent is recovered.
- The filaments are cold drawn to increase fibre orientation 3 to 10 times based on end-use.
- The fine filaments are drawn and wound around spools for further use.
Variations in the basic process that have been developed to meet specific needs change the fiber both in appearance and in certain properties.
Flow Chart of Manufacturing Orlon Acrylic Fiber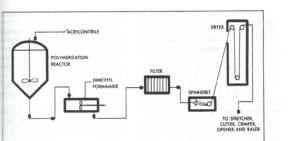
3.3 Yarn Formation:
After orientation and stabilization, the Orlon filaments are shipped as tow and are cut into the staple lengths for spinning into the yarn. The fibers are identified according to type and have particular characteristics suitable for particular purposes. These varieties provide Orlon fibers with considerable flexibility in application to serve numerous consumer uses. The acrylic filaments produced are made into the Spun yarn or High Bulk yarns according to the end requirements.
3.4 Fabric Formation:
Due to the stretch created in the yarn, acrylic fibers are most commonly used in knit wear. It also gives warmth, is comfortable, draplable, retains shape and provides cover in the fabric. Orlon acrylic is also used in weaving of the fabric.
4. EVALUATING FABRICS OF ORLON ACRYLIC:
4.1 PHYSICAL PROPERTIES
| Structure | Acrylic fibre is rod like with uniform diameter when viewed longitudinally under microscope. Longitudinal striations are found parallel to the fibre axis. The cross sectional shape is ‘dumbbell’. The delustered fibre shows spitted appearance. |
| Density | Acrylic has a low density of 1.16 gm/cm. This helps in excellent covering power in fabrics. |
| Strength | The tenacity of acrylic fibres is medium ranging from 2.2 to 2.6. The fibres loose strength when wet to a considerable extent. Compared with the natural fibers, it is weaker than all with the exception of wool. Since Orlon is used primarily as a replacement for wool, its greater strength is an advantage. Even though the fibre is not very strong, it is possible to produce fabrics that satisfy the consumer. |
| Elasticity | Elongation at break of acrylic fibre is medium. The fibres show high elastic recovery of 90-95 % for small extensions. The recovery from higher extension decrease considerably. |
| Resiliency | Acrylic fibres have moderate and good resilience. Fabrics resist wrinkling and undesirable creases hang out easily and quickly. Bulky fabrics are more resistant and lofty. |
| Drapability | The drapability of Orlon varies with the type of fiber. Generally speaking, it provides satisfactory draping qualities. |
| Abrasion resistance | Acrylic has moderate abrasion resistance. |
| Moisture Regain | Acrylic fibre is also a hydrophobic fibre like polyester. It has a regain of 1.0 to 2.5 %. The absorbency if spun acrylic fabrics happen to be better than filament fabrics. The lower absorbency gives resistance to all types of stains. |
| Dimensional Stability | Acrylic fabrics show good dimensional stability in normal use. |
| Shrinkage | The Orlon fiber will shrink in processing and that this characteristic is utilized in obtaining desired effects, such as high-bulk yarns and pile fabrics. However, once the finished product reaches the consumer, it may be expected to have excellent dimensional stability since the Orlon fiber will have practically no further shrinkage. |
| Cleanliness and Washability | These fabrics do not soil or stain easily, and wash or dry cleaning quickly renews their freshness. A mild detergent should, however be used in laundering since strong detergents will damage them. If desired, ordinary cleaning fluid and household bleach may be safely used. |
4.2 THERMAL PROPERTIES:
| Heat conductivity | Acrylic fibre like other synthetics does not conduct heat well. Due to its dumbbell cross sectional shape, it facilitates more bulkiness in fabrics which results in providing warmth and therefore suitable for winter wear. |
| Burning Properties | The fibres shrink away from flame and take up the flame readily and burn faster after removal from flame, leaving a brittle hard black bead. The melting starts at 2320C to 2550C. Ironing below 1600C is recommended. |
4.3 CHEMICAL PROPERTIES:
| Effect of alkalies | Fair to good resistance is found to weak alkalies and strong alkalies at room temperatures. |
| Effect of acids | Acrylic is highly resistant to strong organic as well as mineral acids. |
| Effect of bleaches | Acrylic fabrics can be safely bleached with any household bleach. |
4.4 MISCELLANEOUS PROPERTIES:
| Effect of Sunlight | Acrylic has excellent resistance to sunlight. |
| Effect of Moths and Mildew | Acrylic has excellent resistance to the growth of mildew and all types ofmoths and insects. |
| Effect of Perspiration | Perspiration does not deteriorate the acrylic fabric. |
| Electrical Conductivity | Acrylic is a poor conductor of electricity. |
5. FINISHES GIVEN TO ACRYLIC:
- Heat Setting- for permanent shape retention.
- Antistatic finish- to avoid static build up on fabric.
- Water repellency – for special purpose
- Napping – to produce napped surface and giving warmth.
6. CONSUMER PREFERENCE:
- Acrylics provide good warmth for winter clothing. It is possible and hence preferred to produce light weight bulky fabrics.
- Economical: When compared to wool, it is economical; hence replaced wool to a greater extent in the apparel and rugs and other warm clothing.
- Easy care characteristics.
- Spun acrylic blends are highly preferred for knitting industry.
7. USES:
Acrylic is most important use in the apparel for pile fabrics; thick, snuggly fun furs that are used for coats, jackets, or very warm linings.
Acrylic is used in home furnishings. Upholstery fabrics are the first important use. These are flat woven or velvets. They are also used in blankets, as cost is lower, the bulky fabrics are lighter in weight and the care is easier than for wool blankets. Carpets and Rugs account for the third important use of acrylic in the homes.
They are also used in the industrial uses for their chemical resistance and good weathering properties make them suitable – awnings, tarpaulins, luggage, boats and other vehicle covers, outdoor furniture, tents, office room dividers and bags.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- https://en.wikipedia.org/wiki/Acrylic_fiber
- http://www.aksa.com/en/products/what-is-acrylic-fiber-/what-is-acrylic-fiber-/i-56
- https://www.textileschool.com/121/acrylic-fibres-manmade-artificial-fibres/
- www.fibersource.com/fiber-products/acrylic-fiber/
- http://www.fibersource.com/fiber-products/acrylic-fiber/
- https://nptel.ac.in/courses/116102026/39
ASSIGNMENTS
- Identify textile products made of different acrylic fibers
- Discuss the difference between wool and acrylic apparel products
- Why wool is replacing by acrylic? Discuss.
TYPES OF YARNS
1. INTRODUCTION:
There are different types of yarns and their selection depends on the end use of the product. Each yarn has its own characteristics that vary as per the construction and treatment given in the manufacturing process.
2. DEFINITION:
A yarn is a continuous strand of textile fibers, filaments, or material in a form suitable for knitting, weaving or otherwise intertwining to form a textile fabric – ASTM 1984
Yarn is the generic name for the assemblage of fibers that is laid down or twisted together
3. CLASSIFICATION OF YARNS:
3.1 ACCORDING TO LENGTH OF FIBERS PRESENT IN YARN:
Yarns can be broadly classified as staple-spun yarns or continuous filament yarns. Spun yarns consist of staple fibers assembled and bound together by twist to produce the required characteristics such as strength, handle and appearance.

(Source: Norma, 1988)
3.1.1. SPUN YARNS:
Spun yarns are made from staple fibers and are characterized by protruding fiber ends. Yarn strength is dependent on the cohesiveness of the fibers and on the points of contact resulting from pressure of the twist. The greater the number of points of contact, the greater is the resistance to the fiber slippage within the yarn.
When such properties like absorbency, bulk, warmth, or cotton like or wool like textures are desired in the fabrics, spun yarns are used. As the fiber ends hold the yarn away from close contact from the skin, a spun yarn is more comfortable on a hot humid day than a fabric of smooth filament yarns. Protruding ends contribute to a dull fuzzy appearance, to the shedding of lint, to the formation of pills on the surface of the fabric. They soil readily and give more cover.
3.1.2. FILAMENT YARNS:
The range of filament yarns are as diverse as that of spun staple yarns. The filaments yarns are divided into two types viz., Flat continuous filament and Textured continuous filament yarn.
(a) Continuous filament yarns are produced from long continuous filaments. Filament yarns are primarily man-made. Regular or conventional filament yarns are smooth and silk like as they come from the spinneret. Their smooth nature gives them more luster than spun yarns, but the luster varies with the amount of the de-lustering agent used in the fiber spinning solution and the amount of twist in the yarn. Filament yarns have no protruding ends, so they do not shed lint; they resist pilling; and fabrics made from them tend to shed soil and have less cover (less opaque).
(b) Textured continuous yarns are man made continuous filament yarns that have been modified by processes to introduce crimps, coils, loops or other distortions into the filament or with high twist or low twist. The addition of twist increases bulk. Texturing gives slippery filaments the aesthetic property of spun yarns by altering the surface characteristics and creating space between the fibers. It also improves the thermal and moisture absorption of filament yarns.
3.2. ACCORDING TO THE NUMBER OF PARTS PRESENT IN YARN:
3.2.1. SIMPLE YARNS:
A simple yarn is alike in all its parts. It can be described as spun or filament yarn based on the length of fibers present. A simple yarn also can be described by the number of parts it has, by the direction and amount of twist in the yarn and by the size of the yarn.
Simple yarns are classified as single, ply, and cord.
3.2.1.(a) A single yarn is the product of the first twisting operation that is performed by the spinning machine. Spun, filament and textured yarns are each examples of simple, single yarns.
3.2.1.(b) A ply yarn is made by a second twisting operation, which combines two or more singles. Each part of the yarn is called a ply. Plying tends to increase the diameter, strength and quality of the yarn. Ply yarns are commonly used in the warp direction of woven fabrics to increase the strength of the fabric. These ply yarns are used in men’s shirts women’s sweaters. Two ply and three-ply yarns are found in sewing threads.
End uses for plied yarns:
|
2-fold |
3-fold |
Multi-end |
| Poplins Voiles Gabardines Crepes Lace Sewing thread |
Sewing threads Industrial yarns Canvas Conveyor belts Hosiery |
Industrial yarns Braids Electrical insulation Shoe laces Embroidery |
(Source : Wynne)
3.2.1.(c) A cord/ cable is made by a third twisting operation, which twists ply yarns together. Some types of sewing threads ands some ropes belong to this group. Cords are seldom used in apparel fabric, but used in industrial weight fabrics
End uses for cord yarns
|
Fine |
Coarse |
| Fine sewing threads Crochet yarns Nets |
Industrial cords Heavy swing threads Tyre cords Nettings Fishing lines |
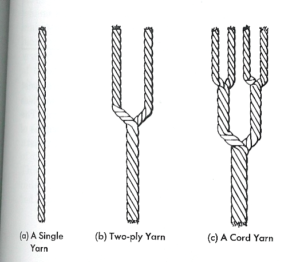
3.2.2. DOUBLE YARNS:
This consists of two or more single strands treated as one in the weaving process, but the strands are not twisted together. These are used for ornamental effect as the low twist yarns produce luster and softness.
3.2.3. NOVELTY YARNS/ FANCY YARNS
May be defined as yarns that are irregular at regular intervals. They may be single, plied, or cord may be spun, filament, or textured combination of yarn types. These yarns are called as novelty yarns, because of their appearance; they lend an interesting or novel effect to fabrics made with them. Fancy yarns have been more common in drapery and upholstery fabrics than apparel fabrics.
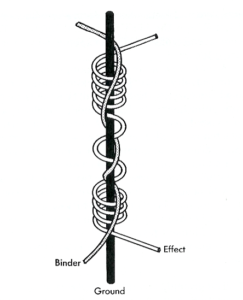 Novelty yarns are made with twisters with special attachments that allow loose, curled, twisted, or looped areas in the yarn. Novelty yarns are usually ply yarns. If novelty yarns are used in only one direction, they are usually in the filling direction. Novelty yarns interest to the plain weave fabrics at lower cost than if effects were obtained from weave. Novelty yarn effects are permanent.
Novelty yarns are made with twisters with special attachments that allow loose, curled, twisted, or looped areas in the yarn. Novelty yarns are usually ply yarns. If novelty yarns are used in only one direction, they are usually in the filling direction. Novelty yarns interest to the plain weave fabrics at lower cost than if effects were obtained from weave. Novelty yarn effects are permanent.
Novelty yarns are usually composed of – a base or ground, an effect, a tie or binder yarn. Base yarn controls length and stability of end product. Effect yarn forms the design or effect. Tie yarn holds effect yarn so that it will remain in position.
3.2.4 TYPES OF NOVELTY YARNS:
- Slub yarn: This is a thick and thin yarn made by varying the amount of twist in the yarn at regular intervals. They are found in drapery and upholstery fabrics.
- Flock yarn: These are frequently called as Flake yarns. These are usually single yarns in which small amounts of fibers either different colours or luster or both are inserted into the yarn and held in place by twist of base yarn. Eg., tweed fabric. This gives a spotted and short streaky appearance.

- Thick and thin yarns: These are similar to slub yarns but these are made from filament fibers unlike slub preparation from staples. The pressure forcing the spinning solution is varied the filament are thick in some places and thin in some.
- Boucle yarn: These are characterized by tight loops projecting from the body of the yarn at fairly regular intervals. They are 3 ply yarns. The effect yarns forms irregular wavy surface and binder ties it to the base. It has a twisted core yarn.

- Loop and curl yarn, gimp yarn: Gimp is same as boucle but the effect yarn is regular semi circular appearance, while in loop

- Ratine: Similar to boucle but the loops are close in ratine and the loops are also smaller and are securely twisted. These yarns have rough surface appearance. The manufacturing requires 2 steps- after the yarn is first made, it is twisted in the opposite direction to establish the desired effect.
- Snarl yarn or spike yarn: This is made in the same way as loop yarn using a highly twisted effect yarn which forms snarls rather than loops.

- Knop (button) yarn / Knot / Nub / Spot yarn: This features prominent bunches of one or more of the component yarn at regular or irregular intervals. This is made on a special machine that permits the base yarn to be held almost stationary while the effect yarn is wrapped around it several times to build upon enlarged segment with brightly colored fibers added at the enlarged knot.
- Seed or splash: They resemble knop or knot yarns but the knot segments is tiny in seed yarn and elongated in splash yarn.
- Cloud: A two colored yarn, in which both yarns take it in turn to obscure or cloud the other, giving the appearance of an intermittent color change.
- Spiral or cocksrew / Eccentric: It is made by twisting together two piles that differ in size, type, or twist. These two parts may be delivered to the twister at different rates of speed.
- Chenille Yarn: These create special effects. Chenille means caterpillar in French. The yarn has a cut pile effect which is bound to the core on the loom warps are arranged in groups (2-6) which are interlaced in a cross weaving manner. Weft is inserted in normal manner. These are cut into wrap way threads.

- Core and Metallic yarns: Though they do not conform to the strict definition of complex yarns but they have surface design and so are included here.
a. Core spun yarns: In these the foundation yarn is completely encircled or wrapped by a second yarn. Ex: core- rubber wrapped by a second yarn – cotton. This gives comfort and durability. Silk wrapped with gold or silver.
b. Metallic yarn: These are primarily decorative. The plastic coating on it resists tarnishing, but care must be taken while pressing, as pure metals are soft, their thin films are used over a core yarn that has replaced gold and silver. There are 2 methods of pressing: The metal may be encased in a plastic coating of either polyester or cellulose acetate butyrate. Color is applied to plastic coating or directly to the aluminum with adhesive.
4. YARN NUMBER: Yarn number varies and it differs according to the kind of fiber. They include,
4.1 COUNT:
Many weaving yarns and sewing thread are numbered by the cotton system (count). Spun yarn size is referred to as number and is expressed in terms of length per unit of weight. It is an indirect system; the finer the yarn, the larger the number. The count is based on the number of hanks (1 hank is 840 yards) in 1 pound of yarn. In this system the unit of weight is remained constant.
Count = Length / one pound,
Count = No. of hanks X 840 yards / one pound.
4.2. DENIER:
Filament yarn size is dependent partly on the size of the holes in the spinneret and partly on the rate at which the solution is pumped through the spinneret and the rate at which it is withdrawn. The size of filament yarns is based on the size of the fibers in the yarn and the number of those fibers grouped into the yarn.
The size of filament yarns is determined as denier, which is expressed in terms of weight per unit of length. If 9000 metes of yarn weigh 1 gram, it is then 1 denier. In this system, the unit of length remains constant. The finer the yarn, the smaller is the number
Denier = weight of yarn in gms/ 9000 meters
1 denier – 9,000 meters weigh 1 gram
4.3 TEX SYSTEM
The International Organization for Standardization has adopted the Tex system, which determines yarn count or number in the same way for all fiber yarns and uses metric units (weight in grams of 1 thousands meters of yarn.)
Tex = weight in gms / 1000 meters of yarn
5. YARN TWIST:
Twist is the spiral arrangement of the fibers around the axis of the yarn. Twist is produced by revolving one end of a fiber strand while the other end is held stationary. Twist binds the fibers together and gives the spun yarn strength. It is a way to vary the appearance of fabrics. The number of twists is referred to as turns per inch. They have a direct bearing on the cost of the yarn because higher twist yields lower productivity.
 5.1 Direction of Twist:
5.1 Direction of Twist:
The direction of twist is described as S-twist and Z-twist, A yarn has S-twist if, when held in a vertical position, the spirals conform to the direction of slope of the central portion of the letter “S,” It is called Z-twist if the direction of spirals conforms to the slope of the central portion of the letter “Z”. Z-twist is the standard twist used for weaving yarns. The majority of single yarns are spun with the twist in the Z direction.
5.2 Amount of twist:
The amount of twist varies with
- the length of the fibers,
- the size of the yarn,
- the intended use.
Increasing the amount of twist up to the point of perfect fiber-to-fiber cohesion will increase the strength of the yarns. Too much twist inserted makes the yarn weaker because of the shearing action that arises as the fibers are at right angles to the axis of the yarn. Fine yarns require more twist than coarse yarns.
Low twist (2-3 turns per inch) in spun yarns results in lofty yarns. This type of twist allows for napping of the fabric.

Average twist is frequently used for yarns made of staple fibers and is very seldom used for filament yarns. The amount of twist that gives warp yarns maximum strength is referred to as standard warp twist.
Hard twist (voile twist) yarns have 30-40 turns per inch. The hardness of the yarn results when twist brings fibers closer together and yarn is more compact. This effect is more pronounced when a twist-on-twist ply yarn is used. This means that the direction of twist in the singles is the same as that of plying twist.

Twist on twist two –ply yarn
Crepe yarns are made of either staple or filament fiber. Crepe is a French word, meaning ‘crinkle’. They are made with a highly twisted yarns (40-80 tpi). This makes the yarn so lively and kinky that it must be twist-set before it can be woven or knitted. Filament crepe yarns are used in fabrics like Georgette and chiffon.
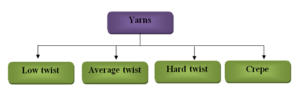
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- Wynne A (1997) Textiles The Motivate Series, Macmillan
- https://youtu.be/v8duRlY6nwA
ASSIGNMENTS
- How textiles are made of yarns? Discuss
- Types of yarn number
- Differentiate between Count, Denier, Tex
- List novelty yarns
FABRIC CONSTRUCTION METHODS
1. INTRODUCTION:
A fabric is a pliable, plane- like structure that can be made into garments and household textiles or used for the industrial purposes. Fabrics are made from the solutions, fibers, yarns, and combinations of these elements with a previously made fabric or cloth. Fabrics are usually available to the consumer by the yards or meters.
The fabric–forming/ construction process determines the appearance and texture, the performance during use and care, and the cost of a fabric. The process often determines the name of the fabric; for example- felt, lace, double knit, and jersey. The cost of fabrics in relation to the construction process depends upon the number of steps involved and the speed of the process; the fewer the steps and the faster the process the cheaper is the fabric. The fabric construction methods include; weaving, knitting, lace making, felts, non-wovens.
2. METHODS OF MAKING FABRICS
2.1 Fabrics made from solutions:
2.1.1 Films: These are made from solutions extruded through narrow slits into warm air or cast into a revolving drum. It is waterproof and resistant to soil. It lacks strength and has low drapability and are weak unless supported by a fabric. Used for shoes, shower curtains, plastic bags, upholstery.
2.1.2 Foam: These are made by incorporating air into elastic like substance. Rubber and polyurethane are most commonly used. Foams are lofty, springy, and bulky. They are too weak to be used without backing. When combined with fashion fabric it gives warmth at low cost. Used in pillows, chair cushions, mattresses, carpet padding and apparel.
2.2 Fabrics made directly from fibres:
2.2.1 Felt: A felt is a fabric made directly from fibres, in the presence of heat, moisture and pressure. Traditionally wool has been used to make felts. Fibre webs of a certain thickness are used. These webs are also called as batt, which are sprinkled with warm water, passed over a steam box and then passed between two rollers. The bats are then drained and allowed to cool off for 24 hrs. The felt is dampened with lubricant like a soap & soda solution and subjected to a pounding process called fulling. Felts do not have grain, do not ravel or fray.
2.2.2. Non Woven fabric: non woven refers to a fibre web structure. A fabric made by bonding or the interlocking of fibres or both accomplished by mechanical, chemical, thermal or solvent means is termed as non-woven fabric. They were first produced in 1930’s. Commercial production began during world war II.
2.2.3 Fabrics made directly from yarns:
2.2.3.1 Weaving: Two or more sets of yarns are interlaced at right angles to each other to make into fabric. This is called as weaving. The fabrics made through weaving are termed as woven fabrics. The yarns running parallel to the length of the fabric are called warp yarns or ends. Those running perpendicular or across to the warp yarns are called weft yarns or picks or filling yarns. Weaving is done on a machine called a loom. Weaving is the most commonly employed technique of making fabric. Different interlacings make different patterns. The fabrics have grain and are rigid when compared to knits.
Grain indicates the direction of the warp or weft yarns. Lengthwise grain is a position along the warp yarns. Crosswise grain is along the filling yarn.
Fabric count or count, is the number of warp and filling yarns per square inch of gray goods. Count is written with the warp number first and followed by weft number. Fabric count is an indication of the quality of fabric – the higher the count, the better the quality for any one fabric and less potential shrinkage and less raveling of seam edges.
Balance is the ratio of the warp yarns to filling yarns in a fabric. A fabric is said to be well balanced if the number of warp yarns and filling yarns are almost equal, For example, a piece of muslin with a thread count of 64 x 60 is considered well balanced.
The lengthwise finished edges where yarns are closely packed are called selvedges. It is the self-edge of a fabric formed by the filling yarn when it turns to go back across the fabric.
 2.2.3.2 Knitting: One or more set of yarns are formed into a series of interlocking loops. It is faster than weaving but requires more yarn for a unit cover. It is stretchy and elastic, porous and resilient. A diverse range of products such as t-shirts, sweaters, socks, undergarments etc are made through knitting.
2.2.3.2 Knitting: One or more set of yarns are formed into a series of interlocking loops. It is faster than weaving but requires more yarn for a unit cover. It is stretchy and elastic, porous and resilient. A diverse range of products such as t-shirts, sweaters, socks, undergarments etc are made through knitting.
A single yarn or several yarns may be used to form the loops by the use of hooked needles. New loops are formed by passing the yarn through previously formed loops. Traditionally, hand knitting is thought to have existed as early as the 5th century. Apart from using knitting needles, commercially knitted fabrics are constructed on knitting machines.
The first real evidence of a knitting machine was the stocking frame invented by the “Reverend William Lee” in 1589. The circular knitting machine and the warp-knitting machine came about 200 years later.
2.2.3.4 Lace: The third basic method of making textiles is lace making. Lace is an open mesh fabric made by intermeshing yarns where in yarns is twisted around each other. The yarns are knotted, interlaced, interlooped and twisted. The fabric has open spaces between and around solid areas of cloth. It can be made by hand or with machine. Patterning can be done in laces using jacquard mechanisms. Laces are used in making apparel (dresses, lingerie), home furnishings (pillows, napkins, kerchiefs, table cloth etc)

2.2.3.5 Net: It is an open-mesh fabric that is held together by knots or thermoplastic yarns that are fused at those points where yarn crosses over. Several types of mesh like square, hexagonal and octagonal shapes are possible. These fabrics are fragile and require care in handling. Application includes veils, curtains, fishing nets, sports equipment, hammocks
2.2.3.6 Braiding: Braids are narrow fabrics in which yarns are interlaced both in lengthwise and diagonal directions. They are pliable, curve around edges nicely and used basically for trims. Braids are essentially of two types: flat braids and round braids. Ex: Cords, shoelaces. Industrially braiding is significant in providing cordage including ropes, hawsers and cables.



2.2.3.7 Crochet: A crochet is used to make fabric from yarns. The yarn is looped around the needles by wrapping it two to three times. It is possible to make different textures and designs.


2.2.3.8 Macramé: A method of making a textile structure by knotting yarns to form geometric patterns



2.4 Composite fabrics:
2.4.1 Coated: A coated fabric combines a textile fabric with a polymer film, which is a semi liquid material that is applied to a fabric substrate. Rubber, Polyvinyl chloride and polyurethane are usually used for coating. Woven, knit or non-woven is used as a base onto which the coating is applied. This provides strength and elongation control. The coating protects from environmental factors like water, chemicals, oil and abrasion. Used for upholstery, handbags, window shades, book covers, shoe liners etc.

2.4.2 Flocked: Short fibres called flock are held on the fabric surface by means of mechanical or electronic bonding.
2.4.3 Foam and Fibre Fabric: Fibres and polyurethane solution are mixed together, cast on a drum or forced through a slit to make fabric. It is napped on both sides. It looks and feels like a suede.

2.4.4 Tufting: Yarns carried by needles are forced through a fabric substrate and cut or left uncut. They are cheaper than woven and knitted pile fabrics. Used widely in rugs, carpets, upholstery etc.
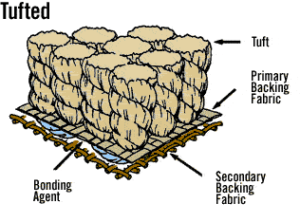
2.5 Multicomponent:
2.5.1 Quilted fabrics are multicomponent fabrics or composite fabrics consisting of three layers : face fabric, fibrefill or batting and backing fabric. These three components are held together by stitch bonding with threads, adhesive or fusing. The wadding can be foam, cotton or fiberfill. They are bulky, warm and decorative. Used in ski jackets, robes, quilts, comforters, upholstery.
2.5.2 Bonded fabrics or laminated fabrics are made from two fabric layers that are adhered together by means of an adhesive. The first bonded fabric was produced in 1958, which was an inexpensive wool flannel bonded to acetate jersey fabric. Two methods of bonding are: wet-adhesive method, flame-foam method. In wet adhesive method, the adhesive is applied to the underside of the fabric. In the foam-flame process, polyurethane foam acts as an adhesive.
The American Society for Testing and Materials (ASTM) defines laminated fabrics as a layered fabric in which the face or surface fabric is joined to a backing fabric with an adhesive that does not significantly to the thickness of the combined fabric.
REFERENCES
- Kaur, N (2011) Fashion Concepts, Comdex: Fashion Design Vol I, Dreamtech Press.
- Hollen & Saddler (1979) Textiles, 5th Edition, Mac Millan Publishing Co.Inc, NY
- https://youtu.be/0fZSTaGvm70
ASSIGNMENT
- What are the method of construction used for producing cleaning scrubs and wipes used at home?
- Uses of non woven
- Production of decorative fabrics? Discuss
- What is grain?
STAIN REMOVAL
1. INTRODUCTION:
A stain is an unwanted discoloration caused by an accident. Stains on clothes have to be removed before laundering otherwise they become permanent and make the clothes unattractive. Fresh stains are easier to remove than dried ones. Most stains are to be cleaned with cold water first. Blood for example should never be cleaned with hot water as heat sets in the stain making it more permanent.
2. TYPES OF STAINS:
Stains are classified as
- Vegetable stains
- Animal stains
- Oil stains
- Mineral stains
2.1 Vegetable stains: These stains are acidic in nature. Hence alkaline medium is most suitable to remove them. Ex: Tea, Coffee, fruits, vegetables, perspiration (fresh stain)
2.2 Animal Stains: These are protein in nature. They get fixed onto the fabric if hot water is used for stain removal. Hence they should always be washed in cold water. Ex: Blood, milk, meat, egg etc.
2.3 Oil Stains: These are easily removed by using soap, solvent or absorbent. Ex: Oil, ghee, butter, cream.
2.4 Mineral Stains: These stains are better treated first in acidic medium followed by alkaline medium. Ex: ink, rust, medicine etc.
3. PRINCIPLES OF STAIN REMOVAL:
- Stains should be removed when they are fresh. They should not be allowed to dry.
- Identify the nature of the stain
- Dilute the stain removing agent in order to avoid damage to the fabric.
- Unknown stain should be first removed with cold water & then with detergent in cold water.
- Acidic stains should be treated with alkaline medium so that a soluble salt is produced which can be leached out easily.
- Stains should be worked out in a circular motion, from outside to inside.
- Work on the wrong side of the stain.
- Stain removing agent should be washed off thoroughly
- Check for color fastness of the fabric before stain removal, by testing at the seam or hem allowance
- Inflammable chemical agents like petrol etc should be used carefully
- While using bleach, bleach the entire garment rather than the stained area to prevent uneven color removal.
4. METHODS OF STAIN REMOVAL:
- Solvent Action: The stained area is dipped in an organic solvent which dissolves the stain.
- Mechanical and emulsifying action: Stained area is sponged or rubbed which dislodges the stain without dissolving.
- Chemical action: Insoluble stain is rendered soluble through oxidation and reduction process. It is later washed out from the fabric.
- Digestion: The stained area is exposed to enzyme which digests it thereby aiding in its removal. Residue can then be washed away.
- Absorption: Absorbents like chalk, corn meal, fullers earth are used in stain removal as they will absorb the liquid staining ingredient like grease, oils etc
5. CLASSIFICATION OF STAIN REMOVING AGENTS:
Stain removing agents can be grouped as
- Solvents: These can either be hydrocarbons (Benzene), chlorinated hydrocarbons (CCl4), petroleum based (turpentine), Alcohols (acetone)
- Bleaches: These can either be oxidizing(H2O2, KMnO4) or reducing( sodium hydrosulphite)
- Emulsifiers: Can either be anionic (soaps) or nonionic (Teepol)
- Enzymes: Proteases, cellulases
- Absorbents: Talcum Powder, French Chalk, Corn Powder, Fullers earth
6. COMMON STAINS AND THEIR REMOVAL
Artificial flowers: Place grubby artificial flowers in a large paper bag add lots of salt and shake vigorously. Then run water through the flowers and watch the dirt just wash away.
Ballpoint ink: Dab the mark with methylated spirit, then rub &rinse.
Blood: A fresh blood stain on clothing can be rinsed out in cold salt water. If the stain is dried bleach with a drop of hydrogen peroxide.
Stained brass: A greener way is to rub with a piece of lemon sprinkled with salt; rinses, dry then rub with olive oil.
Butter: Scrape off as much as one can. Iron with a warm iron between layers of absorbent paper.
Candle wax: Put in a plastic bag in the freezer for an hour or two. Then place fabric between sheets of blotting paper and iron with a warm iron.
Cane furniture stains: Cane furniture can be cleaned and tightened by scrubbing it with hot salted water.
Chewing gum: Put the garment in a plastic bag in the freezer for a while or put an ice pack on the gum. Crack off the solid pieces. Sponge the remainder with dry cleaning fluid.
Chocolate: On clothing, scrape off the solid chocolate with a blunt knife. Pour boiling water from a height or use detergent and work from back of the stain.
Coffee: Sponge stains with borax and pour hot water through the fabric.
Curry: Soak stain with methylated spirit, diluted ammonia or white spirit.
Fly spots: Cold tea will remove fly spots from mirrors.
Hair dye: Rinse fabric immediately with cold water, then wash in warm water with liquid detergent and ammonia.
Perspiration: Stain can be removed by eucalyptus oil with few drops of ammonia.
Iron stains: To clean the outside of your iron, use tooth paste as the iron cools.
Jam stains: Remove jam stains from washable clothes by soaking in a solution of borax and water; then wash as usual.
Lipstick: For lipstick on fabric, try cold water first and, if that fails, put glycerin on the stain, leave overnight, then wash in warm to hot sudsy water.
Ointment stain: Try dry cleaning solution, then rinse in cold water. Then work in liquid detergent and rinse again.
Fig. Stain removing gun used in commercial laundries

REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi.Atma Ram and Sons Ltd
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- https://youtu.be/7Xe7bdmc7lw
- https://www.cleaninginstitute.org/clean_living/stain_removal_chart.aspx
- http://textilelearner.blogspot.com/2014/11/classification-of-stain-and-removal.html
ASSIGNMENTS
- Identify common stains as per their categories
- Discuss the order of stains removal stains
LAUNDRY EQUIPMENT
1. INTRODUCTION:
Laundering is the process of cleaning, washing and finishing clothing and other household clothes and textile items. There are a lot of laundry aids that one can use during the laundering process. The various laundry equipment, their advantages, disadvantages and care are discussed in this chapter.
2. LAUNDRY EQUIPMENT COVERS THE ITEMS USED FOR
- Washing
- Drying
- Finishing
- Storage
2.1 Washing equipment:
Laundry Sink: A laundry sink reduces the amount of work, and labor involved in washing clothes. If constructed at a convenient place, height and in right size it will facilitate washing without causing much strain on the worker. Draining boards should be attached on to either side of the sink. Sink is useful to apply cleansing agents on heavily soiled parts and for stain removal. However, laundering of silks, laces and other delicate items can also be done in a sink.

Boiler: Most of the household clothes such as table and bed linen etc., need boiling in order to disinfect them and to preserve their whiteness. Hence some arrangement for boiling is necessary. For a household, a small round tin of galvanized iron is quite sufficient.
For institutional laundry, a boiler made of copper will be more serviceable and convenient. The material to be used should be hard and rust free. A pair of long tongs or a wooden boiler-stick is necessary for moving the clothes in the boiler and for removing them. Electric boilers are more commonly available in the market.
Tubs and Buckets: Most essential articles as they are used for steeping, washing, rinsing, blueing, starching and carrying the washed linen to the line. In a household, a couple of these are quite sufficient but in an institutional laundry, the minimum number required would be about half a dozen. The most suitable material for tubs and buckets is galvanized iron as it does not readily rust and is easy to clean. Plastic tubs and buckets are more popular and practical.


Enamel Bowls and Basins: Enamel bowls and basins are utilized for several purposes in laundry. Medium size basins are used for washing small articles of silk and wool. These are also used for preparing starch, blue, and dyes. Small bowls are used for stain removal.

Spoons and Containers: Two or three wooden spoons are required for preparing starch, stirring blue and for handling laundry material. Containers such as bottles and jars are needed for storing laundry materials. Metal containers are not advisable as these are liable to react with most of the reagents.
Scrubbing Brushes: Laundering of over soiled clothes and garment parts requires additional brushing or rubbing. Scrubbing brushes with soft and hard bristles are useful. Care should be taken to choose the right bristles to the right fabric.
Scrubbing Boards and Beaters: Most common practice of washing daily wear and heavy household textiles/furnishings at home is by beating them against a stone. Sometimes a wooden beater is also used. This weakens and shortens the life of the fabric. The use of a scrubbing board is an improvement on this method of washing. In western countries, the scrubbing boards are made of corrugated zinc, wood or glass. Beater with a long wooden rod with a disc at one end is suitable for laundering


Use of a Scrubbing Board: The use of a scrubbing board is very simple. A wash tub, half filled with water, is kept on a table 21/2 ft. high. The lower end of the board is placed in the tub. The article to be cleaned is made wet, spread on the board and soap is applied. The article is rubbed up and down on the board. Occasionally, it is dipped in the water to wash out the loosened dirt. The scrubbing board gives efficient work without harming the fabrics. Any cotton fabrics used for every day wear can be washed by this method.
Suction Washer: This consists of two parts. The top part is the wooden handle and the bottom part is the washer. The washer is made of copper and is hollow inside with holes all over its lower portion. Copper is a good and durable material and does not rust easily. Zinc could also be used. A suction washer is used for all types of fabrics, especially for those which need careful handling. All heavy woollens such as blankets, saris, suits, and even delicate fabrics such as laces, silks, and organdies, can be washed by suction washing. Articles to be washed are immersed in soap solution in a tub or a basin and the suction washer is worked up and down on clothes in the soap solution for 15-20 minutes until the dirt is removed.

Dry Cleaning Pump: When dry cleaning is attempted at home, open tubs or basins are used. The solvents used for dry cleaning like petrol and benzene are volatile and evaporate when used in an open basin or a tub. The “Dry cleaning pump” will save petrol as well be efficient. The “Dry cleaning pump” is a round tin with a lid, a tap at the lower side and a fitted in suction washer with a handle. The lid is tightened up by means of three screws which make the tin almost airtight.
When using, the tin should be half filled with petrol, the dirty articles placed in and the lid screwed down. The suction washer is worked up and down by means of a handle for 15 to 20 minutes. Time depends on the amount of dirt in the garment. At the end of the process, the tap is opened and the petrol is received in a bottle through a filter paper. Then the lid is unscrewed and the articles removed from the tin and left to air. The whole apparatus should be made of some rustless metal. A wooden handle to the suction washer is more convenient.
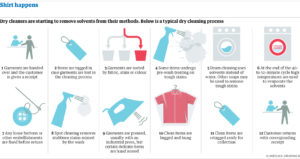
2.2 DRYING EQUIPMENT:
Dryers/ Drying racks: In India, outdoor drying on a clothesline is possible almost all the year round. Clothes pegs are essential and should be stored in a peg bag or box and kept free from dust and stains. In places where outdoor drying is not suitable, drying racks could be used.
There are different varieties of drying racks available in the market. A drying rack with a pulley is convenient as it can be lowered to a level so that a person can spread the garments with ease.
Rods can also be raised and lowered individually in modern drying racks. Portable racks of wood, fibre and steel are also designed in various styles and in various sizes. These racks can be folded after use and hence suit for households with limited space. Another advantage of portable racks is they can be placed out in the sun/open space for drying clothes.
Different types of drying racks



2.3 FINISHING APPARATUS
Ironing is one of the primary finishing operations. Ironing is the process of smoothing out wrinkles by application of heat, pressure and friction, often with application of moisture or steam. Pressing is the act of exerting pressure and heat, with or without steam, to smooth or crease a fabric. It is distinct from ironing by its limitation to special smoothing operations such as crease and pleat setting or touch up of wrinkled areas of a garment.
For ironing, the necessary articles are irons, ironing boards, ironing tables, sleeve boards and coverings for these.
Irons: An Iron is a small appliance, usually electric, for pressing fabrics by hand.
Flat, Charcoal, Electric and Thermostatic irons are different types available in the market.
Charcoal Irons: A charcoal iron consists of a metal hollow box, with a handle at the top. A few pieces of live charcoal are placed inside along with some fresh charcoal and then the iron lid is closed. The draught door at the back is left open to allow the charcoal to continue burning and so heat the iron. As the iron is large & heavy, it is difficult to manipulate. The temperature cannot be regulated. When it gets cold the ash has to be emptied out and the iron has to be filled again with burning charcoal. Another drawback of this iron is that the ash from the coal is likely to fall out and spoil the clothes.

Electric Irons: These are available in various weights and designs. They have nickel or chromium surfaces which are rustproof, hence need no cleaning.
An Electric iron consists of
- The Sole Plate. This is the base of the iron with a highly polished lower surface so that it can glide smoothly over the fabric to be ironed. It may be coated with teflon which prevents sticking as well as corrosion.
- The Element is the most important part in an electric iron. A wire made of an alloy of nickel and chromium is sandwiched between two sheets of mica. The ends of this wire are attached to the plug pins at the rear end of the iron.
- The Press Plate covers the element and has the handle attached to it. It adds weight to the iron. A sheet of asbestos between the element and the press-plate may also be there which prevents the press plate from getting hot.
- The Handle attached to the press plate is of bakelite. Some handles have a thumb rest.
a. Thermostatic Irons

Most electric irons today are thermostatically controlled which helps regulate the temperature of the iron for ironing various fibre fabrics. Table below shows temperatures for ironing various fabrics.
| Fabric | Safe Ironing Temperature |
| Linen | 200°C |
| Cotton | I80°C |
| Wool | I60°C |
| Silk | I60°C |
| Nylon | I60°C |
| Polyester | I60°C |
| Acrylic | 120°C |
| Rayon | 120°C |
| Modacrylic | No Ironing |
b. Steam Irons: These are electric irons having a reservoir into which water is filled. When the current is switched on the water boils and steam is emitted at the ironing surface. This saves time as clothes need not be previously dampened. Most steam irons are combination irons-they give stearn or dry ironing as the user prefers.

Ironing Boards: Ironing board makes the ironing process simpler. It should be well-padded and smooth & covered with a firm white woven cover stretched firmly and fastened well. It should stand firmly. A height of 75 cm is generally comfortable. It has a piece of asbestos at the right hand side on which the hot iron can be safely placed. Ironing boards are collapsible, easy to be moved when not in use and do not need much storing space.
These are also known as skirt-boards.


Sleeve board
It is good to use a sleeve board to facilitate the pressing of sleeves. Thin white muslin cloth is necessary when pressing wool or silk.
Sleeve boards (wooden and padded)


2.4 STORING EQUIPMENT:
Laundry bins/containers: Soiled clothes are stored until they are washed. Store the clothes in dry condition to avoid growth of mould and mildew. Bins are made of steel, wood and plastic are sturdy and durable. Portable laundry bags of canvas and net are also available.




REFERENCES
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi. Atma Ram and Sons Ltd
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- https://www.iso.org/obp/ui/#iso:std:iso:6330:ed-3:v1:en
ASSIGNMENTS
- List the laundry equipment
- Discuss household equipment used
- Best and easy available laundry equipment. Discuss
DRY CLEANING
1. INTRODUCTION:
Dry-cleaning was formerly known as French cleaning or chemical cleaning. It is defined as the cleaning of fabrics in a non-aqueous medium. The dirt in ordinary washing is removed by emulsification and saponification of the grease, where as dry-cleaning indicates the removal of grease by the solvent action of certain liquids and by dry powders which act as grease absorbents.
Dry cleaning may be done by the use of a) absorbents b) grease solvents
Dry cleaning is suitable for removing grease from all kinds of material for cleansing light coloured fabrics that are evenly soiled, and for articles such as furs and dark coloured gloves that cannot be cleaned by solvents alone.
2. DRY CLEANING OPERATIONS: Dry cleaning involves the following operations
a. Marking – soiled garments are marked with a tag, generally a white fabric piece, with a number and some code printing/written with ink. This tag is secured firmly to the garment.
b. Sorting – tears or seams are mended. Later the clothes are grouped into the following groups
- White and light colored clothes
- Dark colored clothes
- White and light colored woolens
- Dark colored woolens
- Draperies and furniture covers
- Raincoats and wind cheaters
At this stage, pockets are checked and cleaned, fancy buttons and buckles are removed.
c. Pre-spotting – heavily soiled garments are treated with solvents. Various stains are removed with different agents.
- Oil stains – volatile dry solvents
- Paints and varnish stains – non-volatile stains
- Water soluble stains – emulsifying agents
- Food stains – enzymes
Pre-spotting is carried out by using a spotting gun which uses compressed air.
d. Cleaning – the dry cleaning cylinder takes a load of about 45 kgs. Delicate clothes are put in a net bag. Appropriate solvent is circulated through the clothes from 5 to 45 minutes depending on the fabric type. The dirt is loosened by the immersion and agitation of the garments.
e. Extraction – the excess solvent is extracted through centrifugation
f. Drying – garments are dried in drier
g. Filtering and distillation of the solvent – as solvents are expensive; they are filtered, distilled and re-used.
h. Inspection – the dried garments are inspected to check if they are clean
i. Finishing – the garment is given a finish that brings it back to its original size, shape
j. Packing – detached buckles and buttons are fixed back, the garment packed for delivery.
3. DIFFERENT ABSORBENTS USED: Starch, magnesium carbonate, French chalk, fuller’s earth, bran, moong powder, bread crumbs, and other commercial dry-cleaning powders. These absorbents are useful for cleaning grease marks on light coloured fabrics, white lace, white furs, white shawls, and white felt hats.
4. METHOD OF ABSORBENT USE
- Shake or brush off loose dirt from the garment.
- Spread a thick layer of the absorbent powder over it and rub it lightly in a circular motion.
- Leave for half an hour to allow the powder to absorb the grease.
- Heat the above powder to dry them and improve their absorbing power
- Rub well into the articles being cleaned.
- Shake and brush out until all the absorbents is removed.
Advantages
- They absorb and clean the grease marks and leaves no sweal or ring on the fabric.
- They are suitable for spotting.
4.1 Grease solvents
- In-flammable: aviation petrol, benzene
- Non-inflammable: carbon tetrachloride, benzene and trichloroethylene and tetra chloroethane
- Dry cleaning soaps or spirit soaps: Dry cleaning soaps are available and it increases the cleansing power of dry cleaning solvents. A small amount should be prepared over the grease spots and very solid parts, before the garment is immersed for cleaning.
- Mineral turpentine is good solvent for grease particularly paint stains.
4.2 Method of use inflammable solvents
The work must be done out of doors or in some place where there is good cross ventilation. The machine contains an inner container, which holds the petrol and the garment. It is closed up and set in motion, after which it is taken out, rinsed and treated as follows.
- Brush the garment well to remove all dust.
- Take sufficient petrol in bowls to clean the garment by hand squeezing, or by the use of suction washer After cleaning, squeeze out as much petrol as possible, wrap in a dry towel or cloth and beat.
- Hang outside to dry for one day to remove the odour.
- Press when thoroughly dry.
- Leave the used petrol in a covered bowl to allow the dirt to settle. After settling the petrol should be carefully poured off the top, and the sediment thrown in open ground.
5. ADVANTAGES OF DRY-CLEANING:
- Dry cleaning is possible for many fabrics for which washing is not suitable such as fur, felt dark-skin gloves
- Crepe fabrics can be cleaned by dry cleaning. This causes no shrinkage as water does.
- Velvet and other pile fabrics can be easily dry cleaned. It will not flattened by dry cleaning.
Dry cleaning is the best method of cleaning any garments with pleats; it does not remove the pleats.
6. DISADVANTAGES
- It is costly.
- The smell of inflammable solvents remains in the clothing for a long time especially for woolens.
- The solvent only acts on dirt held by grease and so stains left after cleaning must be spotted with water, e.g. perspiration, tea, coffee, lemonade stains.
REFERENCES
- Corbman B. P (1983) Textiles-fiber to fabric, Mc Graw-Hill lnt., USA
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi. Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- https://youtu.be/FzmQ6G2aDtw
- https://en.wikipedia.org/wiki/Dry_cleaning
ASSIGNMENTS
- Locate dry cleaning centres at your palce
- Find the tariff for various dry cleaning products
- How dry cleaning is done? Discuss
WATER
1. INTRODUCTION:
Water is a prime agent in laundry process. It is an excellent solvent that removes the soluble and insoluble dirt and dust from the clothes. Water used for laundry should be clean, soft, free from odour, discoloration, iron, organic matter.
Water is obtained from different sources. Generally water is treated to be free from harmful substances in towns and cities. But in most of the villages water is untreated and may cause problems even in laundering. There are three main sources of water:
- Rain water
- Surface water from oceans, rivers, streams, tanks, ponds & lakes
- Sub Soil water or ground water from shallow wells, deep wells, springs etc.
2. TYPES OF WATER
Water is described as being ‘hard’ if it contains more than 60 ppm (parts per million) of calcium and Magnesium. If the mineral content is in the range of 61 to 120 ppm, then it is moderately hard. If it exceeds 180 ppm, then it is described as ‘very hard’.
Water is described as being ‘soft’ if it contains less than 60 ppm (parts per million) of Calcium and Magnesium.
Hard water makes laundering much more harder as the lime salts decompose the soap and form an insoluble soap, which collects as curd, on the surface of water. The detergent action of the soap will only help in precipitating calcium and magnesium (lime salts) since all the soap is utilized in decomposing of lime salts in water. There will not be enough detergent action left in the soap to cleanse the fabric. Thus the fabrics become yellow and pose difficulty in washing. Clothe swashed in hard water develop a grey look and become harsh and uncomfortable to wear.
3. TYPES OF HARDNESS:
The hardness of water can be either
- Temporary or
- Permanent
Temporary Hardness is due to the presence of bicarbonates of calcium and magnesium in water. It can easily be removed by boiling. Permanent Hardness is caused due to the presence of chlorides and sulphates of calcium and magnesium. These mineral salts do not respond to boiling.
4. SOFTENING OF WATER: The process of the removal of hardness from water is called softening. It is highly desirable to soften water with more than 5 ppm for laundry purposes.
4.1 Removal of temporary hardness: It can be removed by the following methods:
- Boiling: During boiling, the bicarbonates of Ca and Mg decompose into insoluble carbonates and give CO2. The insoluble carbonates can be removed by filtration.
- Clark’s method : This process is used on a commercial scale. In this process, calculated amount of lime [Ca(OH)2] is added to temporary hard water.
4.2 Removal of permanent hardness: Permanent hardness can be removed by the following methods:
- Washing soda method: In this method, water is treated with a calculated amount of washing soda (Na2CO3) which converts the chlorides and sulphates of Ca and Mg into their respective carbonates that get precipitated.
- Permutit method: This is a modern method employed for the softening of hard water. Permutit is also known as Zeolite. They are capable of exchanging ions reversibly.
- Ion-exchange Process: This is most modern method for softening hard water. Almost all salts can be removed completely from hard water and the water obtained is as good as distilled water. In this process two types of resins are used i.e. cation exchange resin & anion exchange resin, cation exchange resin contains (-COOH, -SO3H) function groups and are capable of exchanging their H+ ions with cations. While anion exchange resin contains (-NH2, -OH) functional group and are capable of exchanging OH- ions with anions. In the process two columns, one consist of cation exchange resin & another consist of anion exchange resin are used. The hard water is first allowed to pass through a column containing cation exchange resins, which removes all the cations like Ca+2, Mg+2 etc. and release H+ ions.
5. EFFECT OF HARD WATER IN BOILER
Hard water when used for boiler, causes the following troubles:
- Scale or sludge formations
- Priming and foaming
- Caustic embrittlement
- Corrosion.
REFERENCES
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi.Atma Ram and Sons Ltd
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- http://textilelearner.blogspot.com/2014/04/water-consumption-in-textile-industry.html
ASSIGNMENTS
- Discuss various types of water and its effect on detergents’ action in laundry
- Methods of softening water
CLEANSING AGENT – DETERGENTS
1. INTRODUCTION:
‘Detergent’ originates from the verb “to deterge” meaning ‘to clean’. They include laundry soaps, synthetic washing powders, hand cleaners, toilet soaps etc. Detergents contain
- Surfactants which increase the wettability of the fabrics
- Builders and boosters that increase the cleaning power
- Anti redeposition agents that suspend the dirt in the wash water and prevent the redeposition on the clothes
- Enzymes that attack the stains, whiten and reduce the pills
- Optical brighteners etc
Soaps are manufactured from natural oils or fats. Synthetic detergents are made from chemicals. They are available in cake, bar, flakes, powder and liquid form. Naturally occurring detergents like reeta nut, shikakai are ideal for washing wool and silks.
2. COMPOSITION OF SOAPS AND DETERGENTS:
A soap is a metallic salt of a saturated and unsaturated higher fatty acid. It is a product of reaction of an alkali with oils and fats, which is known as saponification. Soaps are anionic in nature.
|
SOAP |
DETERGENT |
| Soaps are alkali salts of higher fatty acids | Detergents are sodium salts of sulphated long chain alcohols |
| Can be used in soft water | Can be used in soft and hard water |
| Dissolve well in hot water | Dissolve well in cold and hot water |
| Not suitable for all kinds of clothing | Since it is not an alkali it is suitable for most fabrics |
| Form less foam | Form more foam |
Two important properties of soap that plays an important part in its cleansing action are;
- its power to lower the surface tension of water
- its property of emulsifying oils and fats.
Soaps and detergents are composed of three parts:
- The active ingredient – obtained from natural oils, fats
- Builders – add bulk to the detergents and are inorganic compounds like carbonates, silicates, phosphates or organic compounds.
- Additives – include bleaching agents, bluing agents, fluorescent brightners, anti-corrosion agents.
2.1 The active ingredient:
All detergents are surfactants (surface active agents), they have the ability to lower the surface tension of water. Surfactants are of four types:
- Anionic Compounds
- Cationic compounds
- Amphoteric compounds
- Non-ionic compounds
2.2 Builders: It is defined as an ingredient which has no surface active property but it increases the bulk and efficiency(detersive power) of the detergent. It may be organic or inorganic in nature. The builders are
A. Inorganic builders: This category includes
- Sodium sulphate: It improves the foaming property of the detergent and gives the powder free flowing property.
- Phosphates: The phosphates soften the water, add alkalinity to the detergent, disperse the soil.
- Sodium silicate: It is generally used as a filler. These help in preventing corrosion of the manufacturing plant and facilitate extrusion of synthetic laundry bars by plasticizing the mass.
- Carbonates: The various carbonates used are soda ash, sodium carbonate, sodium bicarbonate. The most used is soda ash which provides high alkalinity and softens hard water by precipitating calcium and magnesium ions
B. Organic Builders: These are employed in smaller quantities than the organic builders. They include CMC (Carboxy Methyl Cellulose), PVP etc.
2.3 Other Additives:
Optical brightness: It is added to all washing powders. These compounds have the property of absorbing UV rays and convert them into visible blue light there by making the fabrics look brighter and whiter.
Photo Activated Bleaches: These bleaches are absorbed by the fibres from the wash water and get activated in the presence of sunlight thereby bleaching the fabric.
Chelating agents: These compounds bind the Ca and Mg ions in the hard water.
Hydrothropes: They are short chain compounds like Toluene, xylene etc that hold all the materials in liquid detergents.
Enzymes: Enzymes like proteases, amylases and lipases are added to the detergents. Proteases acct on the protein stains, lipases act on grease stains, amylases act on starch.
3. PHENOMENA OF DETERGENCY:
A good detergent should be able to accomplish the following:
- Wet the fabric (wetting action)
- Detach the dirt from fabric surface (emulsifying action)
- Prevent re-deposition of soil on the surface (suspending action)
3.1 Wetting action: Fabric immersed in detergent solution is more readily wetted out than when it is immersed in water alone. Detergent molecules arrange themselves at the water interface thereby lowering the surface tension of water.
3.2 Emulsifying action: The hydrophobic tails of the detergent penetrate into the oil, englobe it and lift it off the fabric surface.
3.3 Suspending action: Dirt englobed by the detergent is held in a stable suspension in water and prevents contact with other surface. Hence the dirt does not get redeposited on the fabric surface.
Hence a good detergent should be able to clean clothes without much agitation, in all types of water, is effective over a wide range of temperatures, is harmless to the skin & fabric, is easily rinsed out and is biodegradable.
REFERENCES
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi. Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- http://textilelearner.blogspot.com/2013/09/list-of-chemicals-used-in-garment.html
- https://youtu.be/VMrbF-vlyvI
ASSIGNMENTS
- Identify textile cleaning agents
- Discuss the types of textile cleaning agents you are using in household laundry
ADDITIONAL LAUNDRY REAGENTS
I. ALKALINE AGENTS
- Washing Soda – Sodium carbonate Na2Co310H2O
This is most commonly used in laundry work and is available in the form of soda crystals which readily dissolve in boiling water. When combined with it improves the detergent power of the solution. It is used to soften water, emulsify grease, removes vegetable or slight scorched stains. It is hard on the skin.
- Borax – Sodium tetra borate – Na2S410H2O
It occurs naturally and it is a white powder. It is a mild alkaline substance readily soluble in cold water. It also used to neutralize acids in stain removal. It prevents scorching or browning of the starch at higher temperature.
- Ammonia – Ammonium HydroxideNH4OH
Ammonia has pungent vapour which may cause a chocking sensation. It is stronger alkali that causes yellowing of silk and wool and bleaching colours, tenders the fabrics. It is used for treating grease and slight scorched stains on animal fabrics, it capable of removing the smell left after using Javelle water. It is also used to neutralize acids.
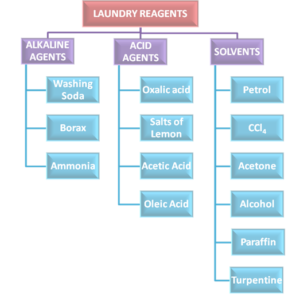
II. ACID AGENTS
- Oxalic acid: It is purchased in the form of white crystals.
Uses
- To remove iron mould and obstinate fruit stains
- To bleach the brown stain after the use of KMnO4
- As a cleanser for white straw hats.
- Oxalic acid has a strong action, should be neutralized by the use of borax or ammonia to prevent damage to fabrics.
- Salts of Lemon COOH COOK: A compound of potassium oxalate and oxalic acid. It is similar to oxalic acid and is employed in the same way and in the same proportions.
- Acetic Acid: Acetic acid is one of the most important acids; household form of acetic acid is vinegar. Glacial acetic acid is strongest and purest; it will not be used in metal vessels. So use in glass enamel or earthenware vessel.
Uses
- It is used as neutralizing agent and removes over blueing.
- It gives an added brightness to the fabrics.
- Acetic acid is a great aid in the finishing of cellulose acetate.
- Oleic Acid, Olein: This belongs to the class of chemicals known as fatty aids. It produces soap when mixed with an alkali. It is used for the spotting of grease and oil stains caused by machinery. This agent is used for treating cotton and linen. It readily felts wool, tends to discolor silk and is unsuitable for colored fabrics.
III. SOLVENTS
Appropriate solvents can be applied to the most delicate fabrics either to remove stains or to dry clean. They do not injure the fibres or the colours of the fabrics.
- Cleaning Benzene or Petrol: It is highly flammable. It is of great value for removing stains containing grease.
- Carbon tetra chloride CCl4: It is more expensive than cleaning benzene, but similar in its action and has great advantage of being non-inflammable. It is extremely volatile and evaporates quickly. It is solvent for paint can be used on all fabrics.
- Acetone: It is useful for many stains. It is highly inflammable on fibres other than cellulose acetate and it is an effective spotting agent for stains caused by cosmetics, nail polish and lipstick, paint and varnish and shoe polish.
- Methylated spirit (Alcohol): It is coloured violet and is poisonous to drink. It can be used to remove sealing wax, silver nitrate and other silver stains. Alcohol dissolves acetate but it can be used on all fabrics.
- Paraffin: Most of the supply of paraffin now comes from the refining of petroleum. Paraffin is employed for removing grease and paint stains on rubber fittings in laundry appliances.
- Turpentine C10H16: It is more expensive than paraffin. It has a distinctive smell, is inflammable and volatile. It acts as a solvent for grease, varnishes, and paint printer’s ink. It is useful for cleaning rubber rollers. It is safe on acetate fabric. It’s one disadvantage is its odour and it can be removed by dry cleaning.
ABSORBENTS
Suitable for removing grease from all fabrics and for general treatment of light coloured fabrics that are evenly soiled. E.g. common salt, bran, fullers earth, powdered magnesia, French chalk, and bread crumbs.
IV. BLEACHES
Bleaching is the process that is capable of whitening fabrics, removing stains, disinfect and deodorize the clothes. Bleaching agents are chemicals which are divided into two classes:
- Oxidizing bleach: it provides oxygen, which combines with the stain to form a colourless compound. All fibers are readily affected by oxidization; so oxidizing bleach must be in contact with a fabric only until the stain is removed. Longer contact will weaken the fabric.
- Reducing bleach: are those, which are capable of removing oxygen from the coloring matter in the stain.
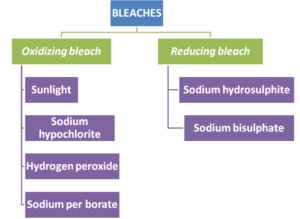
Oxidizing bleaches:
- Open air and sunlight: The world’s oldest method of bleaching is that of treating fabrics in the open air. In many parts of India the fabrics are still spread out on grass, sprinkled with water and left in the open, exposed to rain and dew until they are bleached. Sunlight bleaching can be used for stain removal from bleached cotton and linen fabrics.
- Sodium hypochlorite bleach or Javelle water:
- Lb washing soda
- 1/4 lb chloride of lime
- 1 quart boiling water
- 2 quarts cold water
Method of preparation and use: Dissolve washing soda in boiling water. Mix the chloride of lime with the cold water, allow it to settle, strain off the clear liquid without stirring. Mix together the dissolved washing soda and the filtrate from the chloride of lime. Allow the precipitate of calcium carbonate that is formed to settle. Strain off the clear liquid and store in dark coloured bottles as it is unstable to light. This liquid called Javelle water is sodium hypochlorite, which readily gives off nascent oxygen, a powerful bleaching agent.
It should be used for bleaching white cottons and linens: it must never be used on any other fabrics. Use half Javelle water and half hot water. Leave the stain in the bleach till it is removed. Do not allow the article to soak for more than twenty minutes. To hasten the action, a few drops of vinegar may be added to the bath. Rinse very thoroughly: never allow the bleach dry into the fabric. A small amount of ammonia in the rinsing water will help to remove the smell of the bleach from the fabric.
Cautions:
- Do not use on silk or wool as these fabrics are dissolved in Javelle water.
- Do not use odd coloured fabrics, as many dyes are not fast to chloride bleaches.
- Do not boil fabrics in the solution as it may weaken the material.
3. Hydrogen peroxide bleach: Hydrogen peroxide is effective bleach, not harmful to most fabrics. It can be used in various concentrations, depending upon the amount of bleaching required. One pint to a gallon of water is an average quantity. Rinse thoroughly. A teaspoonful of concentrated ammonia solution or sodium per borate added to each gallon of the solution makes the action stronger.
4. Sodium per borate bleach: This is formed from borax, caustic soda, and hydrogen peroxide. Sodium per borate, which is used in many oxygen-washing powders, dissolves in water making an alkaline bleaching solution, which contains hydrogen peroxide.
Preparation of bleach: One ounce of sodium per borate is dissolved in one gallon of water. If animal fabrics are to be treated, the solution is neutralized with acetic acid, and then, to assist the bleach, it is made slightly alkaline with a little ammonia.
Method and use: One teaspoonful to one pint of water.
- Articles are steeped in the bleach for a few minutes. In the case of animal fabrics, a blood heat solution are used, ½ oz of potassium permanganate dissolved in one gallon of water. For cotton and linen the solution is hot, 1oz of agent in the same quantity of water.
- The bleach is rinsed out of the fabric, which has now stained the characteristic brown colour.
- The article is dipped in one of the following solutions until the staining disappears, which should happen almost at once: (a) sodium hydrosulphite (see below) , (b) oxalic acid 1 oz to 1 gallon of water , or (c) 2- volume hydrogen peroxide , acidified with acetic acid, 1 teaspoon of vinegar to 1 pint of bleach.
- Thorough rinsing must fellow.
Reducing bleaches:
- Sodium hydrosulphite: This is a valuable agent for bleaching all fibres. It is particularly useful for wool and silk, which cannot be treated with sodium hypochlorite.
Action of bleach: The sodium hydrosulphite takes oxygen out of the stain, especially when dissolved in hot water, and becomes sodium Meta bisulphate, which when exposed to air is split up into sodium sulphite and sulphur dioxide, the latter substance being itself a reducing agent.
Method of use:
The storage of sodium hydrosulphite calls for some attention. It must be air- tight, free from moisture and away from heat; otherwise it tends to decompose owing to absorption of oxygen. In extreme circumstances it may take fire, hence work should be carried out near an open window if possible, since the sulphur dioxide, which is given off, is pungent and cause irritation to the throat and lungs.
The bleach can be used in concentrated form for spotting or in solution. Hydrosulphite solutions find application in the spotting treatment for many stains due to grass, dung, hard court, leather, boot polish, mildew, coloured ink, potassium permanganate and dye stains. For bleaching in solution, the articles are steeped for a few minutes in a bath containing 1 to 4 teaspoons of the bleach to 1 pint of cold, hot or boiling water according to the resistance of the stain to be removed and the nature of the fibre. The article must be rinsed thoroughly in water containing a high concentration of soap .If a stain is being treated on an article which is coloured in part, it may happen that the bleach accidentally runs into the colour and changes it. Immediate dipping in an alkaline solution of the immediate application of soap may rectify the trouble. When colour is taken out by a reducing agent, sponging with acetic acid can restore it.
Care must be taken to see that it must be used in contact with metal, for this causes a black stain on all fabrics. Only vessels of wood or earthenware should be used.
- Sodium bisulphate: This is a mild reducing agent, which results from the partial neutralization of sulphurous acid by caustic soda. Its bleaching action is due to the fact that it yields sulphur dioxide, which takes oxygen out of the stain.
Method and use:
It must be used in the proposition of 2 tablespoonful’s to 1 of water, Neutralization or thorough washing must follow, otherwise sulphuric acid will appear in the fabric through the action of oxygen.
Sodium thiosulphate: (hypo) for cottons
¼ oz sodium thiosulphate
/8 oz 36%acetic acid
2 quarts water
Over bleaching:
The over bleaching of cotton and linen goods during laundering is one of the main causes of general weakness of the fabrics. The fibres become brittle and harsh, and give a distinct crackle when rubbed together.
How to avoid over bleaching
Use a bleach of known strength. Never exceed strength of 5 grains per gallon. Keep the temperature below 1400 always measure quantities of bleach accurately; dilute bleach and add gradually. Although most over bleaching is due to the use of chlorine bleaches, it must be understood that all the oxidizing agents have the same effect on cotton and linen. It is a fallacy to consider that hydrogen peroxide is safe bleach. Chlorine bleach should not be applied at too high temperature. It should not exceed 160 Degree Fahrenheit.
REFERENCES
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi. Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
ASSIGNMENTS
- List the suitable reagents for different textile fibers
- Discuss the best suitable reagent for bed linen
TEXTILE CARE LABELS
1. INTRODUCTION:
There has been an extensive growth in textile fibres. Market is inundated with a large variety of textile fibers. This has often resulted in a single garment being made from different fiber types. Such garments require special care in buying and laundering which has increased the significance of care labels and tags.
To assist consumers in getting information about clothing care, the Federal Trade Commission in 1971 issued the Care Labeling Rule. This Rule requires manufacturers and importers to attach care instructions to garment. When you purchase clothing items, hangtags (tags) and labels are attached to them. They give information about products. This might include the size, price, special features, brand name, fiber content, finishes, manufacturer, country of origin, and care instructions. Hangtags and labels exist to identify products, to help sell them, to help consumers make decisions about them and explain proper care. They have been developed through joint efforts of federal government.
2. LABELS USED IN THE CLOTHING INDUSTRY:
A label is defined as that part of a product, which carries information about the product or the sellers. Labels in the clothing industry provide guidelines to the consumer about the quality and care of the product.
Label, are permanently attached to garments on the inside where they do not show during wear. They are usually made of ribbon or cloth. They may be attached at the back neckline or waistline, on a facing, or at a side seam. It has even become fashionable to put labels on the outside of sportswear garments. Jeans often have leather like printed patch sewn on the outside. They may be any color or style as long as they do not ravel. They must give certain types of information. The information may be written on both sides of a label if the label is attached so it can be turned over. Sometimes label information is stamped onto shirttails or collars with indelible ink, or glued or fused onto fabrics. Law requires most of the information on labels.
Fig. Labels

3. USES OF CARE LABELS:
Care labels have many benefits mainly for the consumer:
- Reliable care labels prolong the life if the product
- Care labels help consumers in making buying decisions that are based on their awareness of the materials (fibers, fabrics).
- Care labels ensure that the appearance and performance of the product are maintained through proper cleaning process.
- Besides this care labels add value to the products.
- Care labels have to be printed at least in three different languages, especially when the products are for overseas markets. This requires more space and oversized labels.
4. ASTM CARE LABEL SYSTEM:
The ASTM (American Society for Testing Materials) care labeling committee has developed care symbols in lieu of words on an apparel care label. The ASTM care symbols are simple, comprehensive and easy to understand. They have been harmonized with care labeling instructions and international symbol systems.
The ASTM care symbol system uses six basic symbols to depict textile care instructions on a label. The five symbols for the basic care processes are:
- The washtub (washing)
- Triangle (bleaching)
- Square (drying)
- Iron (ironing and pressing)
- Circle (dry cleaning)
- The sixth symbol is ‘X’ is placed over the other symbols and indicates ‘Do Not’
4.1 Washing symbols:
The washtub is used to represent the washing process. The machine cycle is placed under the tub and the water temperature is indicated by dots the Celsius symbol inside the tub. Other wash symbols include a hand in the tub to indicate hand washing and a twisted cloth with an “X” on its means do not wring. Dots have been used to indicate the water temperature. ‘X’ on the washtub indicates do not wash.
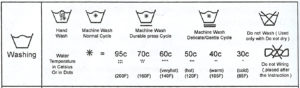
4.2 Bleaching symbols:
The triangle represents bleaching. The plain triangle means any bleach when needed. The shaded triangle means only non-chlorine bleach and the shaded triangle with an “X” through it means do not bleach.

4.3 Drying symbols:
The square represents during tumble-drying and air-drying. The square with a circle inside depicts tumble-drying. Dots and underlines represent the tumble-drying, heat settings and machine cycles. There are also other symbols for tumble dry no heat (a solid circle) in the square and for tumble dry any heat (an empty circle in the square)
Air-drying is indicated by symbols inside the square. A clothesline in the square means line dry, three vertical lines in the square mean drip dry and a horizontal line in the square means dry flat. If diagonal lines are placed over a symbol, it means dry in the shade.

4.4 Ironing symbols:
The iron represents iron or press steam may also be used to indicate it.
An “X” on the iron represents no iron, no steam.

4.5 Dry clearing symbols:
The circle represents the process of dry cleaning. The additional four symbols are short cycle, low moisture, no steam finishing and low heat. One of the issues related to dry cleaning is the accurate reporting of the solvent used in dry cleaning. Any new solvents used in dry cleaning can be reported by these symbols. ‘X’ symbol on circle indicates do not dry clean the fabric.

5. TAGS:
Hangtags printed-paper tags, may be hung from the garment by plastic staple, barb, adhesives or string. Hangtags are designed to draw attention to the garment and are hung on the side of the garment so that the customer can see them easily. These are detachable “signs,” on the garments. They are often hung from buttons, buttonholes, zippers, belt loops, or underarm seams. They are removed before garments are worn.
In general, hangtags tell consumers what manufacturers want to say about their products. Much of that information is voluntary (not required by law). Hangtags are a form of advertising or promotion to help sell products. Information about performance features, special finishes, reinforced pockets, adjustable button cuffs, reversibility, etc. is usually provided on hangtags. Symbols and logos are often displayed to identify the designers, manufacturers, or sellers. Sometimes a certification or seal of approval tells of good test results from a lab. Guarantees may assure replacement or money refunded if items do not perform satisfactorily.
Fig. Tags

REFERENCES
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi.Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- Vatsala, R. 2003. Textbook of Textiles and Clothing. New Delhi. Indian Council of Agriculture Research
- https://www.omo.com/za/laundry/laundry-tips/fabrics/wash-care-symbols.html
- http://www.generallabel.com/clothing-labels/
- https://youtu.be/tkZe8pRkie0
- https://youtu.be/jf81pHWJrH4
ASSIGNMENTS
- Identify textile care labels and tags
- Discuss the types of care label and tags
- Describe ant three care labels and tags used by national and internals brands
STORING OF CLOTHES
1. INTRODUCTION:
Moths and other insects can attack clothes made from animal fibres. The amount of destruction of these garments by moths is so high that one has to take steps to prevent it.
Care of Garments
2. CARE OF CLOTHES
- Brush garments thoroughly and often keep them free from dust. Empty out pockets. Shake well before brushing. Sun and air kill the grubs and keep the moths away.
- Garments which have been worn should not be put away until they have been thoroughly aired. Cup boards, boxes and clothes-closets should be aired frequently.
- Washable garments should be laundered frequently. Garments which cannot be laundered should be dry cleaned occasionally.
- Suits and overcoats should be hung away on clothes hangers.
- Light often fades the colour of fabrics, hence protect them with covers made for that purposes. Keep such garments in dark closets which can be frequently aired.
- Do not put away garments in a damp condition. Moist atmosphere causes mildew, which penetrates into the fibre changes its colour and may even cause it to fall to pieces.
- Protect textiles from destructive insects. The grubs feed upon feed upon wool fabrics, carpets, furs and feathers. The moth is gray in colour.
3. MUST TO FOLLOW:
3.1 Spraying: Wool and its storage place may be sprayed with a fluid or dust insecticide in which D.D.T is incorporated to give it a measure of permanent protection obtained.
3.2 Repellent: Repellents such as tobacco, dried neem leaves, cedar chips, camphor, and moth balls are used. Naphthalene flakes are more efficient than the traditional moth balls. Paradichlorobenzene is the best repellent.
3.3 Packing: Pack away all woolens and furs wrapped in newspapers as the moth dislikes printers ink. The box may be lined and covered with tarred paper.
3.4 Fumigation: It is a poisonous gas e.g. hydrocyanic acid. It destroys grubs and moths.
3.5 Addition of an insecticide to the wool: This entails adding substances to the wool which either poisons the larvae or renders the wool indigestible.
4. DISINFECTIONS IN THE HOME
Disinfection of clothing in necessary in the home as a precaution against the spreading of infectious disease by bacteria or other organisms
Fresh air, sunlight, dryness, and cleanliness are conditions in which bacteria are less likely to be prevalent clothing to be disinfected can be divided into two groups.
- Those can be disinfected by boiling
- Those that must be disinfected by means other than boiling.
4.1 . Disinfections of Clothing by Boiling
This method is suitable for all bleached cottons and linen and would be the method used to disinfect bed linen and personal garments of suitable materials. Home treatment for infected clothing would be follows.
Clothing should be steeped for 12 hours immediately it has been from use.
Carbolic acid – 1 table spoon – 1 quart
Lysol – 1 table spoon – 3 quart
Izal – 1 table spoon – 1 gallon
Dettol – 1 table spoon – 1 quart
Wash by mechanical means, put into the boiler in softened soapy water, and allow coming to boiling point and boiling for 1 hour. Rinse very thoroughly. Dry out of doors. Clothing treated like this should be free from any infection.
4.2 Disinfection of the clothing that cannot be boiled may be treated as follows
- By spraying with a solution of 1 tablespoonful of formalin in a pint of warm water.
- By pouring half pint of formalin over 50 oz of potassium permanganate in a metal dish and leaving surrounded by the clothing in a closed room for 5-6 hours.
- D.D.T is an insecticide useful for delousing, mothproofing, and for the destruction of other undesirable insects on clothing or in store places.
REFERENCES
- Dantyagi, S. 1959. Fundamentals of textiles and their Care. New Delhi. Orient Longman Limited.
- Deulkar, D. and Tarabai.1967. Household textiles and laundry Work. 3rd ed. Delhi. Atma Ram and Sons Ltd
- Kadolph S J (2013) Textiles: Pearson New International Edition, Pearson Education Ltd, Asia
- Noemia, D Souza (1998) Fabric Care , New Age Publications
- https://youtu.be/nJAYRXjZoMY
ASSIGNMENTS
- List the suitable disinfecting agents used for for different textile fibers
- Discuss the best suitable practice for storing clothes at hostel shelves
FOLDING AND PACKING OF CLOTHES
1. INTRODUCTION:
Make your closet shelves appear as crisp and tidy to avoid unwanted creases, stains and maintain the quality of the apparel products. Folding and packing also helps in saving lot of time in rush hours. Always try to utilize space in storage area to the maximum level by arranging apparel items separately like shirts, pants, sweaters, socks, and underwear.
2. GENERAL TIPS
- Use ALL available space, but keep it organized.
- Keep out-of-season (winter/summer) clothes out of the way.
- More likely to wear something means it is seen commonly.
- Keep 1-2 hooks for quick-n-dirty clean up products
- Don’t forget the backs of deep shelves, and high-up places.
3. HANGING CLOTHES
- Iinstal a second closet rod above main one. This basically doubles your hanging space.
- Matching hangers makes everything look organised.
- Cascading hooks will work with almost any hanger.
- Tiered hangers are good for small apparel products.
4. FOLDING CLOTHES
Items that are best folded on shelves are jeans, sweaters ladies tops, skirts etc. Use shelf dividers to keep folded piles from toppling over. Folding tips for different apparel items are given below,
4.1 Small items: Socks: Even though rolling them will keep them together, they lose elasticity this way. Instead, lay two single socks flat, one on top of the other, and simply fold them in half.
Panty hose and stockings: These are best rolled from the feet toward the waistband and folded over the opening to keep them from unraveling.
Tip: Drawer dividers: Keep socks corralled while leaving space for other items in the same drawer.
4.2 Linen Closet
Towels: Best folded lengthwise into thirds and then folded in half, twice. That way the rounded edge faces out without being bulkier than the middle.
Storing linens: There are two ways in a set – fitted and flat sheet with the matching pillowcases or like with like -all of the pillowcases stacked together, for example.
4.3 Clutches and Purses
Empty the contents of purse before it’s stored. Crumbs can attract bugs, pens can leak and batteries might corrode. Not to mention the extra weight of a purse might wear out a handle when it’s hung.
Purses: If the space on shelf available, display purses on a shelf with tissue paper stuffing. The paper will keep their shape and help them sit better.
Clutches: They’re best filed within the constraints of a shelf divider, open bin or drawer, since they’re flat.
4.4 Dreeses: Hanging Out
Pants: They’ll keep their crease best if hung lengthwise from the bottom.
Blouses or T-shirts: If they’re made of thin materials that prove challenging to fold, they always get hung in my closet.
REFERENCES
- https://lifehacker.com/how-to-organize-a-lot-of-clothing-in-very-little-closet-1516664381
- https://www.forbes.com/sites/houzz/2013/03/20/best-storage-secrets-for-clothes/#5557a9e71498
ASSIGNMENT
- Discuss the difficulties in storing clothes and tips to avoid them

